Nicotinic acetylcholine receptors modulate osteoclastogenesis
- PMID: 26970742
- PMCID: PMC4789270
- DOI: 10.1186/s13075-016-0961-x
Nicotinic acetylcholine receptors modulate osteoclastogenesis
Abstract
Background: Our aim was to investigate the role of nicotinic acetylcholine receptors (nAChRs) in in-vitro osteoclastogenesis and in in-vivo bone homeostasis.
Methods: The presence of nAChR subunits as well as the in-vitro effects of nAChR agonists were investigated by ex vivo osteoclastogenesis assays, real-time polymerase chain reaction, Western blot and flow cytometry in murine bone marrow-derived macrophages differentiated in the presence of recombinant receptor activator of nuclear factor kappa B ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). The bone phenotype of mice lacking various nAChR subunits was investigated by peripheral quantitative computed tomography and histomorphometric analysis. Oscillations in the intracellular calcium concentration were detected by measuring the Fura-2 fluorescence intensity.
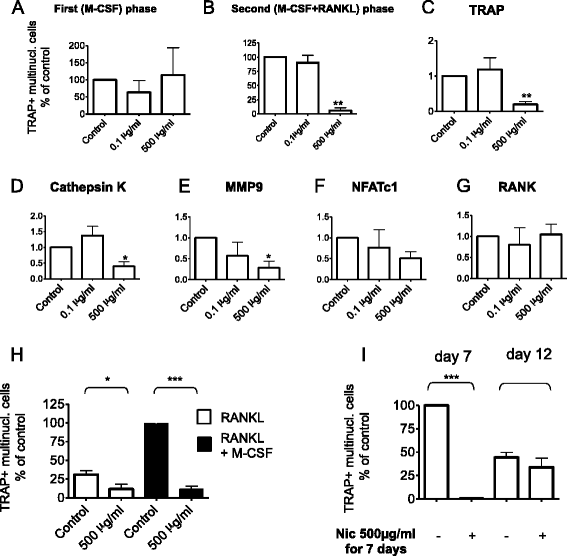
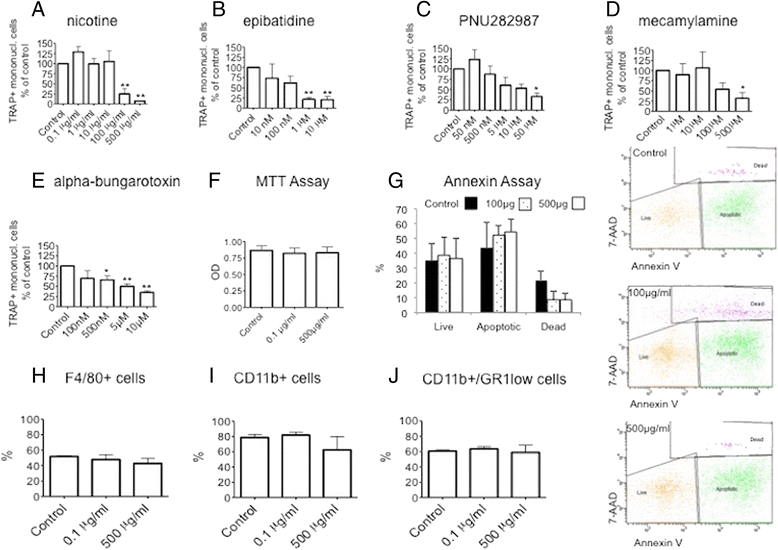
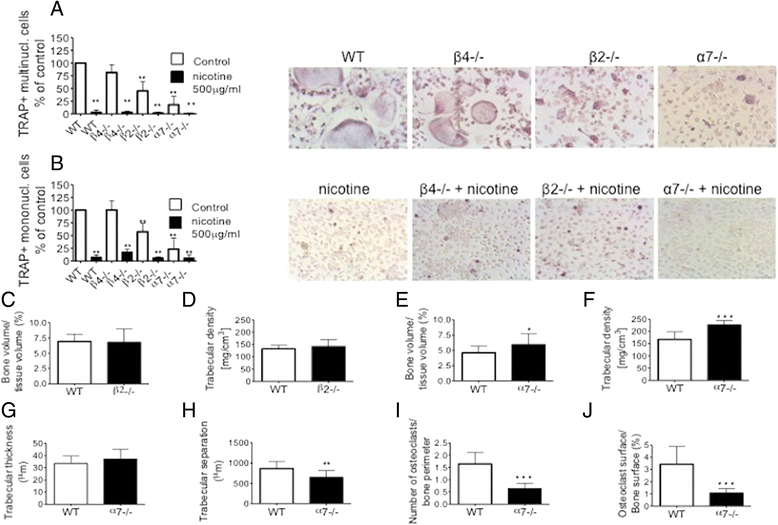
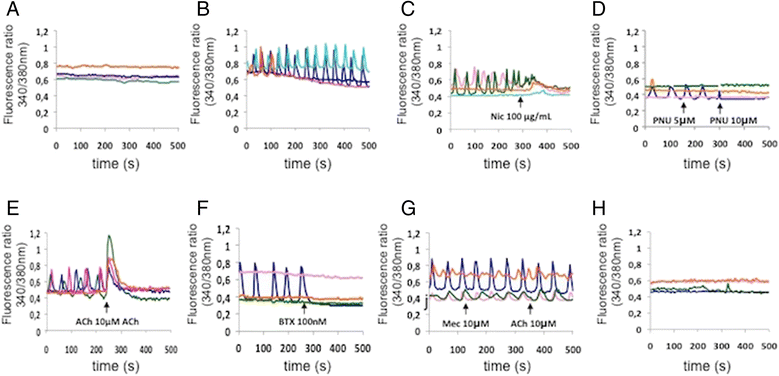
Results: We could demonstrate the presence of several nAChR subunits in bone marrow-derived macrophages stimulated with RANKL and M-CSF, and showed that they are capable of producing acetylcholine. nAChR ligands reduced the number of osteoclasts as well as the number of tartrate-resistant acidic phosphatase-positive mononuclear cells in a dose-dependent manner. In vitro RANKL-mediated osteoclastogenesis was reduced in mice lacking α7 homomeric nAChR or β2-containing heteromeric nAChRs, while bone histomorphometry revealed increased bone volume as well as impaired osteoclastogenesis in male mice lacking the α7 nAChR. nAChR ligands inhibited RANKL-induced calcium oscillation, a well-established phenomenon of osteoclastogenesis. This inhibitory effect on Ca(2+) oscillation subsequently led to the inhibition of RANKL-induced NFATc1 and c-fos expression after long-term treatment with nicotine.
Conclusions: We have shown that the activity of nAChRs conveys a marked effect on osteoclastogenesis in mice. Agonists of these receptors inhibited calcium oscillations in osteoclasts and blocked the RANKL-induced activation of c-fos and NFATc1. RANKL-mediated in-vitro osteoclastogenesis was reduced in α7 knockout mice, which was paralleled by increased tibial bone volume in male mice in vivo.
Keywords: Acetylcholine receptor; Nicotine; Osteoclastogenesis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

