Quantitative mass spectrometry reveals changes in SNAP-25 isoforms in schizophrenia
- PMID: 26971072
- PMCID: PMC5017887
- DOI: 10.1016/j.schres.2016.03.002
Quantitative mass spectrometry reveals changes in SNAP-25 isoforms in schizophrenia
Abstract
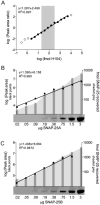
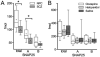
SNAP-25 and syntaxin are presynaptic terminal SNARE proteins altered in amount and function in schizophrenia. In the ventral caudate, we observed 32% lower SNAP-25 and 26% lower syntaxin, but greater interaction between the two proteins using an in vitro assay. SNAP-25 has two isoforms, SNAP-25A and B, differing by only 9 amino acids, but with different effects on neurotransmission. A quantitative mass spectrometry assay was developed to measure total SNAP-25, and proportions of SNAP-25A and B. The assay had a good linear range (50- to 150-fold) and coefficient of variation (4.5%). We studied ventral caudate samples from patients with schizophrenia (n=15) previously reported to have lower total SNAP-25 than controls (n=13). We confirmed 27% lower total SNAP-25 in schizophrenia, and observed 31% lower SNAP-25A (P=0.002) with 20% lower SNAP-25B amounts (P=0.10). Lower SNAP-25A amount correlated with greater SNAP-25-syntaxin protein-protein interactions (r=-0.41, P=0.03); the level of SNAP-25B did not. Administration of haloperidol or clozapine to rats did not mimic the changes found in schizophrenia. The findings suggest that lower levels of SNAP-25 in schizophrenia may represent a greater effect of the illness on the SNAP-25A isoform. This in turn could contribute to the greater interaction between SNAP25 and syntaxin, and possibly disturb neurotransmission in the illness.
Keywords: Neural plasticity; Neurotransmission; Postmortem; SNARE proteins; Synapse.
Copyright © 2016 Elsevier B.V. All rights reserved.
Conflict of interest statement
None of the authors have a conflict of interest relevant to the subject of this paper. In addition to their primary employers: Dr. Clare Beasley received an honorarium from the Ontario Mental Health Foundation. Dr Alasdair Barr has acted as a consultant to Eli Lilly Canada. Dr J John Mann received grants from GlaxoSmithKline and Novartis. Dr Peter Falkai received fees for professional services from Astra Zeneca, BMS, Eisai, Eli Lilly, Lundbeck, Wyeth, and Jansen-Cilag. Dr William Honer has received consulting fees or sat on paid advisory boards for: MDH Consulting, In Silico, Novartis, Lundbeck and Roche; received honoraria from Rush University, the Korean Society for Schizophrenia Research, the Centre for Addiction and Mental Health (Toronto), the BC Schizophrenia Society, the Fraser, Vancouver Coastal and the Providence Health Authorities, and the Canadian Agency for Drugs and Technology in Health; and received grants from the Canadian Institutes of Health Research (CIHR). Drs. Barakauskas, Rosoklija, Ilievski, Stankov, Dwork, Moradian and Morin declare that, except for income received from their primary employers, no financial support or compensation was received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Figures


References
-
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naïve schizophrenic subjects. Biol Psychiatry. 2009;65:1091–1093. - PubMed
-
- Barakauskas VE, Beasley CL, Barr AM, Ypsilanti AR, Li HY, Thornton AE, Wong H, Rosokilja G, Mann JJ, Mancevski B, Jakovski Z, Davceva N, Ilievski B, Dwork AJ, Falkai P, Honer WG. A novel mechanism and treatment target for presynaptic abnormalities in specific striatal regions in schizophrenia. Neuropsychopharmacol. 2010;35:1226–1238. - PMC - PubMed
-
- Barr AM, Young CE, Phillips AG, Honer WG. Selective effects of typical antipsychotic drugs on SNAP-25 and synaptophysin in the hippocampal trisynaptic pathway. Int J Neuropsychopharmacol. 2006;9:457–463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

