Dysfunction of NMDA receptors in Alzheimer's disease
- PMID: 26971324
- PMCID: PMC4917574
- DOI: 10.1007/s10072-016-2546-5
Dysfunction of NMDA receptors in Alzheimer's disease
Abstract
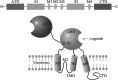
N-methyl-D-aspartate receptors (NMDARs) play a pivotal role in the synaptic transmission and synaptic plasticity thought to underlie learning and memory. NMDARs activation has been recently implicated in Alzheimer's disease (AD) related to synaptic dysfunction. Synaptic NMDARs are neuroprotective, whereas overactivation of NMDARs located outside of the synapse cause loss of mitochondrial membrane potential and cell death. NMDARs dysfunction in the glutamatergic tripartite synapse, comprising presynaptic and postsynaptic neurons and glial cells, is directly involved in AD. This review discusses that both beta-amyloid (Aβ) and tau perturb synaptic functioning of the tripartite synapse, including alterations in glutamate release, astrocytic uptake, and receptor signaling. Particular emphasis is given to the role of NMDARs as a possible convergence point for Aβ and tau toxicity and possible reversible stages of the AD through preventive and/or disease-modifying therapeutic strategies.
Keywords: AD; Extrasynaptic; NMDA receptors; Synaptic transmission; Tripartite synapse.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

