Synaptic organization of striate cortex projections in the tree shrew: A comparison of the claustrum and dorsal thalamus
- PMID: 26971364
- PMCID: PMC5018911
- DOI: 10.1002/cne.23998
Synaptic organization of striate cortex projections in the tree shrew: A comparison of the claustrum and dorsal thalamus
Abstract
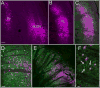
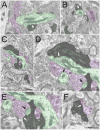
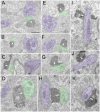
The tree shrew (Tupaia belangeri) striate cortex is reciprocally connected with the dorsal lateral geniculate nucleus (dLGN), the ventral pulvinar nucleus (Pv), and the claustrum. In the Pv or the dLGN, striate cortex projections are thought to either strongly "drive", or more subtly "modulate" activity patterns respectively. To provide clues to the function of the claustrum, we compare the synaptic arrangements of striate cortex projections to the dLGN, Pv, and claustrum, using anterograde tracing and electron microscopy. Tissue was additionally stained with antibodies against γ-aminobutyric acid (GABA) to identify GABAergic interneurons and non-GABAergic projection cells. The striate cortex terminals were largest in the Pv (0.94 ± 0.08 μm2 ), intermediate in the claustrum (0.34 ± 0.02 μm2 ), and smallest in the dLGN (0.24 ± 0.01 μm2 ). Contacts on interneurons were most common in the Pv (39%), intermediate in the claustrum (15%), and least common in the dLGN (12%). In the claustrum, non-GABAergic terminals (0.34 ± 0.01 μm2 ) and striate cortex terminals were not significantly different in size. The largest terminals in the claustrum were GABAergic (0.51 ± 0.02 μm2 ), and these terminals contacted dendrites and somata that were significantly larger (1.90 ± 0.30 μm2 ) than those contacted by cortex or non-GABAergic terminals (0.28 ± 0.02 μm2 and 0.25 ± 0.02 μm2 , respectively). Our results indicate that the synaptic organization of the claustrum does not correspond to a driver/modulator framework. Instead, the circuitry of the claustrum suggests an integration of convergent cortical inputs, gated by GABAergic circuits. J. Comp. Neurol. 525:1403-1420, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: GABA; RRID:AB_2278725; RRID:AB_2333091; RRID:AB_258833; RRID:AB_477329; RRID:AB_477652; RRID:AB_572256; RRID:AB_94259; RRID:nif-000-30467; corticoclaustral; corticothalamic; dorsal lateral geniculate nucleus; pulvinar; retinogeniculate; synapse.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no known conflicts of interest that could inappropriately influence this work.
Figures







References
-
- Bickford ME, Carden WB, Patel NC. Two types of interneurons in the cat visual thalamus are distinguished by morphology, synaptic connections, and nitric oxide synthase content. J Comp Neurol. 1999;413:83–100. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

