Sirt1: Role Under the Condition of Ischemia/Hypoxia
- PMID: 26971525
- PMCID: PMC11482061
- DOI: 10.1007/s10571-016-0355-2
Sirt1: Role Under the Condition of Ischemia/Hypoxia
Abstract
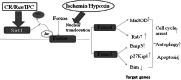
Silent information regulator factor 2-related enzyme 1 (sirtuin 1, Sirt1) is a nicotinamide adenine dinucleotide-dependent deacetylase, which can deacetylate histone and non-histone proteins and other transcription factors, and is involved in the regulation of many physiological functions, including cell senescence, gene transcription, energy balance, and oxidative stress. Ischemia/hypoxia injury remains an unresolved and complicated situation in the diseases of ischemia stroke, heart failure, and coronary heart disease, especially among the old folks. Studies have demonstrated that aging could enhance the vulnerability of brain, heart, lung, liver, and kidney to ischemia/hypoxia injury and the susceptibility in old folks to ischemia/hypoxia injury might be associated with Sirt1. In this review, we mainly summarize the role of Sirt1 in modulating pathways against energy depletion and its involvement in oxidative stress, apoptosis, and inflammation under the condition of ischemia/hypoxia.
Keywords: Aging; Apoptosis; Deacetylase; Ischemia/hypoxia; Oxidative stress; Sirt1.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L (2007) Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev 128:546–552 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

