Presenilin adopts the ClC channel fold
- PMID: 26971579
- PMCID: PMC4918416
- DOI: 10.1002/pro.2919
Presenilin adopts the ClC channel fold
Abstract
Presenilin is an integral membrane aspartate protease that regulates cellular processes by cleaving proteins within the cell membrane. The recent crystal structure of presenilin reveals a conspicuous pore in a bundle of nine α-helices, which was originally thought to adopt a novel protein fold. However, here I show that the presenilin fold is a variant of the ClC chloride channel/transporter fold. This observation may have important implications for presenilin's postulated biological role as a calcium leak channel.
Keywords: ClC channel; calcium leak channel; presenilin; protein fold.
© 2016 The Authors Protein Science published by Wiley Periodicals, Inc. on behalf of The Protein Society.
Figures
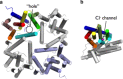

Similar articles
-
Structure of a presenilin family intramembrane aspartate protease.Nature. 2013 Jan 3;493(7430):56-61. doi: 10.1038/nature11801. Epub 2012 Dec 19. Nature. 2013. PMID: 23254940
-
CLC chloride channels: correlating structure with function.Curr Opin Struct Biol. 2002 Aug;12(4):531-9. doi: 10.1016/s0959-440x(02)00358-5. Curr Opin Struct Biol. 2002. PMID: 12163078 Review.
-
Crystal structure of the cytoplasmic domain of the chloride channel ClC-0.Structure. 2006 Feb;14(2):299-307. doi: 10.1016/j.str.2005.10.008. Structure. 2006. PMID: 16472749
-
Biochemical and biophysical approaches to study the structure and function of the chloride channel (ClC) family of proteins.Biochimie. 2016 Sep-Oct;128-129:154-62. doi: 10.1016/j.biochi.2016.08.008. Epub 2016 Aug 21. Biochimie. 2016. PMID: 27554851 Review.
-
The intracellular region of ClC-3 chloride channel is in a partially folded state and a monomer.J Biochem. 2006 May;139(5):813-20. doi: 10.1093/jb/mvj099. J Biochem. 2006. PMID: 16751588
Cited by
-
Presenilin 1 Regulates Membrane Homeostatic Pathways that are Dysregulated in Alzheimer's Disease.J Alzheimers Dis. 2020;77(3):961-977. doi: 10.3233/JAD-200598. J Alzheimers Dis. 2020. PMID: 32804090 Free PMC article. Review.
-
Dysregulation of Intracellular Calcium Signaling in Alzheimer's Disease.Antioxid Redox Signal. 2018 Oct 20;29(12):1176-1188. doi: 10.1089/ars.2018.7506. Epub 2018 Aug 3. Antioxid Redox Signal. 2018. PMID: 29890840 Free PMC article. Review.
-
Interactions of Mitochondria/Metabolism and Calcium Regulation in Alzheimer's Disease: A Calcinist Point of View.Neurochem Res. 2017 Jun;42(6):1636-1648. doi: 10.1007/s11064-017-2182-3. Epub 2017 Feb 8. Neurochem Res. 2017. PMID: 28181072 Free PMC article. Review.
-
Dysregulation of neuronal calcium homeostasis in Alzheimer's disease - A therapeutic opportunity?Biochem Biophys Res Commun. 2017 Feb 19;483(4):998-1004. doi: 10.1016/j.bbrc.2016.09.053. Epub 2016 Sep 15. Biochem Biophys Res Commun. 2017. PMID: 27641664 Free PMC article. Review.
-
Calcium signaling and molecular mechanisms underlying neurodegenerative diseases.Cell Calcium. 2018 Mar;70:87-94. doi: 10.1016/j.ceca.2017.06.008. Epub 2017 Jun 30. Cell Calcium. 2018. PMID: 28728834 Free PMC article. Review.
References
-
- Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B (2002) Identification of signal peptide peptidase, a presenilin‐type aspartic protease. Science 296:2215–2218. - PubMed
-
- Li X, Dang S, Yan C, Gong X, Wang J, Shi Y (2013) Structure of a presenilin family intramembrane aspartate protease. Nature 493:56–61. - PubMed
-
- Wolfe MS (2013) Structural biology: membrane enzyme cuts a fine figure. Nature 493:34–35. - PubMed
-
- Holm L, Sander C (1993) Protein structure comparison by alignment of distance matrices. J Mol Biol 233:123–138. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

