Modulation of anxiety and fear via distinct intrahippocampal circuits
- PMID: 26971710
- PMCID: PMC4816644
- DOI: 10.7554/eLife.14120
Modulation of anxiety and fear via distinct intrahippocampal circuits
Abstract
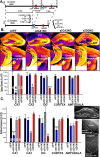
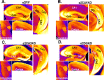
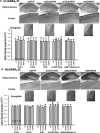
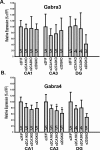
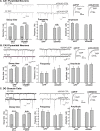
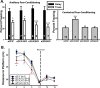
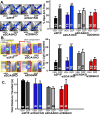
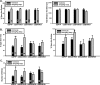
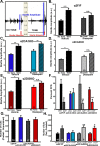
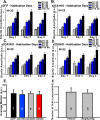
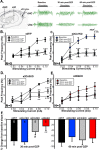
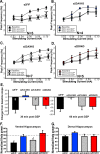
Recent findings indicate a high level of specialization at the level of microcircuits and cell populations within brain structures with regards to the control of fear and anxiety. The hippocampus, however, has been treated as a unitary structure in anxiety and fear research despite mounting evidence that different hippocampal subregions have specialized roles in other cognitive domains. Using novel cell-type- and region-specific conditional knockouts of the GABAA receptor α2 subunit, we demonstrate that inhibition of the principal neurons of the dentate gyrus or CA3 via α2-containing GABAA receptors (α2GABAARs) is required to suppress anxiety, while the inhibition of CA1 pyramidal neurons is required to suppress fear responses. We further show that the diazepam-modulation of hippocampal theta activity shows certain parallels with our behavioral findings, suggesting a possible mechanism for the observed behavioral effects. Thus, our findings demonstrate a double dissociation in the regulation of anxiety versus fear by hippocampal microcircuitry.
Keywords: GABAA receptor; anxiety; diazepam; fear; hippocampus; mouse; neuroscience; theta oscillations.
Conflict of interest statement
The other authors declare that no competing interests exist.
UR: Received compensation for professional services from Concert Pharmaceuticals in the last three years.
Figures
Similar articles
-
Α2 GABAA receptor sub-units in the ventral hippocampus and α5 GABAA receptor sub-units in the dorsal hippocampus mediate anxiety and fear memory.Neuroscience. 2013 Nov 12;252:169-77. doi: 10.1016/j.neuroscience.2013.08.012. Epub 2013 Aug 17. Neuroscience. 2013. PMID: 23962649
-
Modulation of septo-hippocampal Theta activity by GABAA receptors: an experimental and computational approach.Neuroscience. 2004;126(3):599-610. doi: 10.1016/j.neuroscience.2004.03.043. Neuroscience. 2004. PMID: 15183510
-
Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons.J Neurophysiol. 2006 Aug;96(2):846-57. doi: 10.1152/jn.01199.2005. J Neurophysiol. 2006. PMID: 16835366
-
Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information.Hippocampus. 2001;11(5):578-98. doi: 10.1002/hipo.1073. Hippocampus. 2001. PMID: 11732710 Review.
-
Regional dissociations within the hippocampus--memory and anxiety.Neurosci Biobehav Rev. 2004 May;28(3):273-83. doi: 10.1016/j.neubiorev.2004.03.004. Neurosci Biobehav Rev. 2004. PMID: 15225971 Review.
Cited by
-
Loss of calsyntenin paralogs disrupts interneuron stability and mouse behavior.Mol Brain. 2022 Mar 12;15(1):23. doi: 10.1186/s13041-022-00909-8. Mol Brain. 2022. PMID: 35279170 Free PMC article.
-
GSK-3β deletion in dentate gyrus excitatory neuron impairs synaptic plasticity and memory.Sci Rep. 2017 Jul 18;7(1):5781. doi: 10.1038/s41598-017-06173-4. Sci Rep. 2017. PMID: 28720858 Free PMC article.
-
Anxiety-like Behavior and GABAAR/BDZ Binding Site Response to Progesterone Withdrawal in a Stress-Vulnerable Strain, the Wistar Kyoto Rats.Int J Mol Sci. 2022 Jun 30;23(13):7259. doi: 10.3390/ijms23137259. Int J Mol Sci. 2022. PMID: 35806264 Free PMC article.
-
Anxiety and hippocampal neuronal activity: Relationship and potential mechanisms.Cogn Affect Behav Neurosci. 2022 Jun;22(3):431-449. doi: 10.3758/s13415-021-00973-y. Epub 2021 Dec 6. Cogn Affect Behav Neurosci. 2022. PMID: 34873665 Review.
-
Abnormalities of hippocampal shape and subfield volumes in medication-free patients with obsessive-compulsive disorder.Hum Brain Mapp. 2019 Oct 1;40(14):4105-4113. doi: 10.1002/hbm.24688. Epub 2019 Jun 12. Hum Brain Mapp. 2019. PMID: 31188536 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

