Signal, Noise, and Variation in Neural and Sensory-Motor Latency
- PMID: 26971946
- PMCID: PMC4824642
- DOI: 10.1016/j.neuron.2016.02.012
Signal, Noise, and Variation in Neural and Sensory-Motor Latency
Abstract
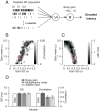
Analysis of the neural code for sensory-motor latency in smooth pursuit eye movements reveals general principles of neural variation and the specific origin of motor latency. The trial-by-trial variation in neural latency in MT comprises a shared component expressed as neuron-neuron latency correlations and an independent component that is local to each neuron. The independent component arises heavily from fluctuations in the underlying probability of spiking, with an unexpectedly small contribution from the stochastic nature of spiking itself. The shared component causes the latency of single-neuron responses in MT to be weakly predictive of the behavioral latency of pursuit. Neural latency deeper in the motor system is more strongly predictive of behavioral latency. A model reproduces both the variance of behavioral latency and the neuron-behavior latency correlations in MT if it includes realistic neural latency variation, neuron-neuron latency correlations in MT, and noisy gain control downstream of MT.
Keywords: Abducens; area MT; correlated variation; floccular complex; neuron-behavior correlations; reaction time; smooth pursuit eye movements.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

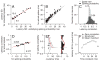
References
-
- Bell AH, Meredith MA, Van Opstal AJ, Munoz DP. Stimulus intensity modifies saccadic reaction time and visual response latency in the superior colliculus. Exp Brain Res. 2006;174:53–59. - PubMed
-
- Bollimunta A, Knuth KH, Ding M. Trial-by-trial estimation of amplitude and latency variability in neuronal spike trains. J Neurosci Methods. 2007;160:163–170. - PubMed
-
- Brown EN, Barbieri R, Ventura V, Kass RE, Frank LM. The time-rescaling theorem and its application to neural spike train data analysis. Neural Comput. 2002;14:325–346. - PubMed
-
- Celesia GG, Puletti F. Auditory input to the human cortex during states of drowsiness and surgical anesthesia. Electroen Clin Neuro. 1971;31:603–609. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

