Coordinated Excitation and Inhibition of Prefrontal Ensembles during Awake Hippocampal Sharp-Wave Ripple Events
- PMID: 26971950
- PMCID: PMC4824654
- DOI: 10.1016/j.neuron.2016.02.010
Coordinated Excitation and Inhibition of Prefrontal Ensembles during Awake Hippocampal Sharp-Wave Ripple Events
Abstract
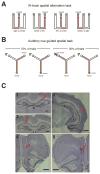
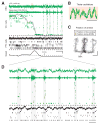
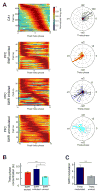
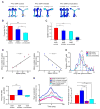
Interactions between the hippocampus and prefrontal cortex (PFC) are critical for learning and memory. Hippocampal activity during awake sharp-wave ripple (SWR) events is important for spatial learning, and hippocampal SWR activity often represents past or potential future experiences. Whether or how this reactivation engages the PFC, and how reactivation might interact with ongoing patterns of PFC activity, remains unclear. We recorded hippocampal CA1 and PFC activity in animals learning spatial tasks and found that many PFC cells showed spiking modulation during SWRs. Unlike in CA1, SWR-related activity in PFC comprised both excitation and inhibition of distinct populations. Within individual SWRs, excitation activated PFC cells with representations related to the concurrently reactivated hippocampal representation, while inhibition suppressed PFC cells with unrelated representations. Thus, awake SWRs mark times of strong coordination between hippocampus and PFC that reflects structured reactivation of representations related to ongoing experience.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Amaral DG, Witter MP. Hippocampal Formation. In: Paxinos C, editor. The Rat Nervous System. Academic Press; 1995. pp. 443–493.
-
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40:177–188. - PubMed
-
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron. 2010;66:921–936. - PubMed
-
- Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

