Nanosecond formation of diamond and lonsdaleite by shock compression of graphite
- PMID: 26972122
- PMCID: PMC4793081
- DOI: 10.1038/ncomms10970
Nanosecond formation of diamond and lonsdaleite by shock compression of graphite
Abstract
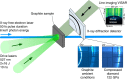
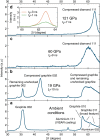
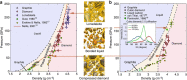
The shock-induced transition from graphite to diamond has been of great scientific and technological interest since the discovery of microscopic diamonds in remnants of explosively driven graphite. Furthermore, shock synthesis of diamond and lonsdaleite, a speculative hexagonal carbon polymorph with unique hardness, is expected to happen during violent meteor impacts. Here, we show unprecedented in situ X-ray diffraction measurements of diamond formation on nanosecond timescales by shock compression of pyrolytic as well as polycrystalline graphite to pressures from 19 GPa up to 228 GPa. While we observe the transition to diamond starting at 50 GPa for both pyrolytic and polycrystalline graphite, we also record the direct formation of lonsdaleite above 170 GPa for pyrolytic samples only. Our experiment provides new insights into the processes of the shock-induced transition from graphite to diamond and uniquely resolves the dynamics that explain the main natural occurrence of the lonsdaleite crystal structure being close to meteor impact sites.
Figures

Similar articles
-
Transformation of shock-compressed graphite to hexagonal diamond in nanoseconds.Sci Adv. 2017 Oct 27;3(10):eaao3561. doi: 10.1126/sciadv.aao3561. eCollection 2017 Oct. Sci Adv. 2017. PMID: 29098183 Free PMC article.
-
Ultrafast transformation of graphite to diamond: an ab initio study of graphite under shock compression.J Chem Phys. 2008 May 14;128(18):184701. doi: 10.1063/1.2913201. J Chem Phys. 2008. PMID: 18532830
-
Sequential Lonsdaleite to Diamond Formation in Ureilite Meteorites via In Situ Chemical Fluid/Vapor Deposition.Proc Natl Acad Sci U S A. 2022 Sep 20;119(38):e2208814119. doi: 10.1073/pnas.2208814119. Epub 2022 Sep 12. Proc Natl Acad Sci U S A. 2022. PMID: 36095186 Free PMC article.
-
Unique Nanomechanical Properties of Diamond-Lonsdaleite Biphases: Combined Experimental and Theoretical Consideration of Popigai Impact Diamonds.Nano Lett. 2019 Mar 13;19(3):1570-1576. doi: 10.1021/acs.nanolett.8b04421. Epub 2019 Feb 14. Nano Lett. 2019. PMID: 30735045
-
Two-Dimensional Diamond-Diamane: Current State and Further Prospects.Nano Lett. 2021 Jul 14;21(13):5475-5484. doi: 10.1021/acs.nanolett.1c01557. Epub 2021 Jul 2. Nano Lett. 2021. PMID: 34213910 Review.
Cited by
-
An approach for the measurement of the bulk temperature of single crystal diamond using an X-ray free electron laser.Sci Rep. 2020 Sep 3;10(1):14564. doi: 10.1038/s41598-020-71350-x. Sci Rep. 2020. PMID: 32884061 Free PMC article.
-
Production of diamond using intense heavy ion beams at the FAIR facility and application to planetary physics.Sci Rep. 2023 Jan 26;13(1):1459. doi: 10.1038/s41598-023-28709-7. Sci Rep. 2023. PMID: 36702850 Free PMC article.
-
Evidence for Crystalline Structure in Dynamically-Compressed Polyethylene up to 200 GPa.Sci Rep. 2019 Mar 12;9(1):4196. doi: 10.1038/s41598-019-40782-5. Sci Rep. 2019. PMID: 30862904 Free PMC article.
-
Fabricating Graphene and Nanodiamonds from Lignin by Femtosecond Laser Irradiation.ACS Omega. 2021 Dec 6;6(49):33995-34002. doi: 10.1021/acsomega.1c05328. eCollection 2021 Dec 14. ACS Omega. 2021. PMID: 34926947 Free PMC article.
-
Stress Writing Textured Graphite Conducting Wires/Patterns in Insulating Amorphous Carbon Matrix as Interconnects.Sci Rep. 2017 Aug 29;7(1):9727. doi: 10.1038/s41598-017-10294-1. Sci Rep. 2017. PMID: 28852077 Free PMC article.
References
-
- DeCarli P. S. & Jamieson J. C. Formation of diamond by explosive shock. Science 133, 1821–1822 (1961). - PubMed
-
- Nemeth P. et al. Lonsdaleite is faulted and twinned cubic diamond and does not exist as a discrete material. Nat. Commun. 5, 5447 (2014). - PubMed
-
- Bovenkerk H. P., Bundy F. P., Hall H. T., Strong H. M. & Wentorf R. H. Preparation of diamond. Nature 184, 1094–1098 (1959).
-
- Erskine D. J. & Nellis W. J. Shock-induced martensitic phase transformation of oriented graphite to diamond. Nature 349, 317–319 (1991).
-
- Hanneman R. E., Strong H. M. & Bundy F. P. Hexagonal diamonds in meteorites: implications. Science 166, 995–997 (1967). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

