Specific interference shRNA-expressing plasmids inhibit Hantaan virus infection in vitro and in vivo
- PMID: 26972493
- PMCID: PMC4820803
- DOI: 10.1038/aps.2015.165
Specific interference shRNA-expressing plasmids inhibit Hantaan virus infection in vitro and in vivo
Abstract
Aim: To investigate the antiviral effects of vectors expressing specific short hairpin RNAs (shRNAs) against Hantaan virus (HTNV) infection in vitro and in vivo.
Methods: Based on the effects of 4 shRNAs targeting different regions of HTNV genomic RNA on viral replication, the most effective RNA interference fragments of the S and M genes were constructed in pSilencer-3.0-H1 vectors, and designated pSilencer-S and pSilencer-M, respectively. The antiviral effect of pSilencer-S/M against HTNV was evaluated in both HTNV-infected Vero-E6 cells and mice.
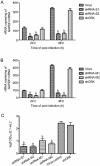
Results: In HTNV-infected Vero-E6 cells, pSilencer-S and pSilencer-M targeted the viral nucleocapsid proteins and envelope glycoproteins, respectively, as revealed in the immunofluorescence assay. Transfection with pSilencer-S or pSilencer-M (1, 2, 4 μg) markedly inhibited the viral antigen expression in dose- and time-dependent manners. Transfection with either plasmid (2 μg) significantly decreased HTNV-RNA level at 3 day postinfectin (dpi) and the progeny virus titer at 5 dpi. In mice infected with lethal doses of HTNV, intraperitoneal injection of pSilencer-S or pSilencer-M (30 μg) considerably increased the survival rates and mean time to death, and significantly reduced the mean virus yields and viral RNA level, and alleviated virus-induced pathological lesions in lungs, brains and kidneys.
Conclusion: Plasmid-based shRNAs potently inhibit HTNV replication in vitro and in vivo. Our results provide a basis for development of shRNA as therapeutics for HTNV infections in humans.
Figures



References
-
- Kruger DH, Figueiredo LT, Song JW, Klempa B. Hantaviruses--globally emerging pathogens. J Clin Virol 2015; 64: 128–36. - PubMed
-
- Maes P, Clement J, Gavrilovskaya I, Van Ranst M. Hantaviruses: immunology, treatment, and prevention. Viral Immunol 2004; 17: 481–97. - PubMed
-
- Kariwa H, Yoshimatsu K, Arikawa J. Hantavirus infection in East Asia. Comp Immunol Microbiol Infect Dis 2007; 30: 341–56. - PubMed
-
- Schonrich G, Rang A, Lutteke N, Raftery MJ, Charbonnel N, Ulrich RG. Hantavirus-induced immunity in rodent reservoirs and humans. Immunol Rev 2008; 225: 163–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

