Medroxyprogesterone acetate levels among Kenyan women using depot medroxyprogesterone acetate in the FEM-PrEP trial
- PMID: 26972780
- PMCID: PMC4894753
- DOI: 10.1016/j.contraception.2016.03.003
Medroxyprogesterone acetate levels among Kenyan women using depot medroxyprogesterone acetate in the FEM-PrEP trial
Abstract
Objective: To describe medroxyprogesterone acetate (MPA) levels among Kenyan depot medroxyprogesterone acetate (DMPA) users in the FEM-PrEP HIV prevention trial, and to compare MPA levels between ARV for HIV prevention (treatment) and placebo groups.
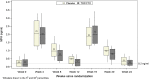
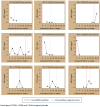
Study design: We measured MPA in previously collected plasma samples from 63 Kenyan trial participants who used DMPA for one or two complete intervals. We separately assessed MPA levels among the nine DMPA users who became pregnant at this site.
Results: Mean MPA levels at the end of each 12week injection interval were 0.37ng/ml (95% CI: 0.25, 1.99) and 0.28ng/ml (95% CI: 0.19, 1.22) among participants assigned TDF/FTC and 0.49 (95% CI: 0.40, 1.27) and 0.39 (95% CI: 0.31, 1.17) among those assigned placebo. The difference between groups was not statistically significant overall, or in an analysis which adjusted for the observed low adherence to TDF/FTC. Unanticipated findings of this analysis were low 12-week MPA levels among DMPA users in both study arms. Of 61 women who contributed data for the first DMPA injection interval, 26.2% had MPA levels<0.1ng/ml and 9.8% had levels below the detection level (0.02ng/ml) at 12weeks post-injection. Levels were similar at the end of the second injection interval. Five of nine women who became pregnant had levels below 0.15ng/mL at the time of their last negative pregnancy test.
Conclusions: Use of TDF/FTC did not appear to affect serum MPA levels, however we found lower than expected MPA concentrations at the end of the dosing interval among DMPA users in the FEM-PrEP trial, the cause of which are unknown.
Implications: This study presents some of the few available data on MPA levels among DMPA users in Africa. The low levels among users described here, together with a number of pregnancies among DMPA users, are potentially concerning and require further investigation.
Keywords: Depot medroxyprogesterone acetate; HIV; Kenya; Preexposure prophylaxis.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- United Nations, Department of Economic and Social Affairs . 2013. Population Division (2013). World Contraceptive Patterns.
-
- Kantorova V. United Nations, Department of Economic and Social Affairs, Population Division; New York: 2013. National, regional and global estimates and projections of the number of women aged 15 to 49 who are married or in a union, 1970–2030.
-
- Malarcher S., Meirik O., Lebetkin E., Shah I., Spieler J., Stanback J. Provision of DMPA by community health workers: what the evidence shows. Contraception. 2011;83:495–503. - PubMed
-
- Land R., Richey C. Baltimore, MD; INFO Project, Johns Hopkins Bloomberg School of Health: 2006. Expanding services for injectables. population reports, series K.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous