Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development
- PMID: 26972897
- PMCID: PMC4925329
- DOI: 10.1016/j.ajog.2016.03.001
Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development
Abstract
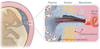
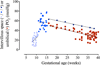
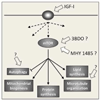
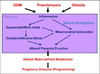
Although much progress is being made in understanding the molecular pathways in the placenta that are involved in the pathophysiology of pregnancy-related disorders, a significant gap exists in the utilization of this information for the development of new drug therapies to improve pregnancy outcome. On March 5-6, 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health sponsored a 2-day workshop titled Placental Origins of Adverse Pregnancy Outcomes: Potential Molecular Targets to begin to address this gap. Particular emphasis was given to the identification of important molecular pathways that could serve as drug targets and the advantages and disadvantages of targeting these particular pathways. This article is a summary of the proceedings of that workshop. A broad number of topics were covered that ranged from basic placental biology to clinical trials. This included research in the basic biology of placentation, such as trophoblast migration and spiral artery remodeling, and trophoblast sensing and response to infectious and noninfectious agents. Research findings in these areas will be critical for the formulation of the development of future treatments and the development of therapies for the prevention of a number of pregnancy disorders of placental origin that include preeclampsia, fetal growth restriction, and uterine inflammation. Research was also presented that summarized ongoing clinical efforts in the United States and in Europe that has tested novel interventions for preeclampsia and fetal growth restriction, including agents such as oral arginine supplementation, sildenafil, pravastatin, gene therapy with virally delivered vascular endothelial growth factor, and oxygen supplementation therapy. Strategies were also proposed to improve fetal growth by the enhancement of nutrient transport to the fetus by modulation of their placental transporters and the targeting of placental mitochondrial dysfunction and oxidative stress to improve placental health. The roles of microRNAs and placental-derived exosomes, as well as messenger RNAs, were also discussed in the context of their use for diagnostics and as drug targets. The workshop discussed the aspect of safety and pharmacokinetic profiles of potential existing and new therapeutics that will need to be determined, especially in the context of the unique pharmacokinetic properties of pregnancy and the hurdles and pitfalls of the translation of research findings into practice. The workshop also discussed novel methods of drug delivery and targeting during pregnancy with the use of macromolecular carriers, such as nanoparticles and biopolymers, to minimize placental drug transfer and hence fetal drug exposure. In closing, a major theme that developed from the workshop was that the scientific community must change their thinking of the pregnant woman and her fetus as a vulnerable patient population for which drug development should be avoided, but rather be thought of as a deprived population in need of more effective therapeutic interventions.
Keywords: drugs; placenta; pregnancy; therapeutics; trial.
Published by Elsevier Inc.
Conflict of interest statement
All other authors report no conflict of interest.
Figures














References
-
- Chaddha V, Viero S, Huppertz B, Kingdom J. Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med. 2004;9:357–369. - PubMed
-
- Redline RW. Placental inflammation. Semin Neonatol. 2004;9:265–274. - PubMed
-
- Andraweera PH, Dekker GA, Roberts CT. The vascular endothelial growth factor family in adverse pregnancy outcomes. Hum Reprod Update. 2012;18:436–457. - PubMed
-
- Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

