Modulation of kidney urea transporter UT-A3 activity by alpha2,6-sialylation
- PMID: 26972907
- PMCID: PMC4945389
- DOI: 10.1007/s00424-016-1802-0
Modulation of kidney urea transporter UT-A3 activity by alpha2,6-sialylation
Abstract
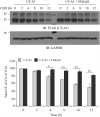
Two urea transporters, UT-A1 and UT-A3, are expressed in the kidney terminal inner medullary collecting duct (IMCD) and are important for the production of concentrated urine. UT-A1, as the largest isoform of all UT-A urea transporters, has gained much attention and been extensively studied; however, the role and the regulation of UT-A3 are less explored. In this study, we investigated UT-A3 regulation by glycosylation modification. A site-directed mutagenesis verified a single glycosylation site in UT-A3 at Asn279. Loss of the glycosylation reduced forskolin-stimulated UT-A3 cell membrane expression and urea transport activity. UT-A3 has two glycosylation forms, 45 and 65 kDa. Using sugar-specific binding lectins, the UT-A3 glycosylation profile was examined. The 45-kDa form was pulled down by lectin concanavalin A (Con A) and Galant husnivalis lectin (GNL), indicating an immature glycan with a high amount of mannose (Man), whereas the 65-kDa form is a mature glycan composed of acetylglucosamine (GlcNAc) and poly-N-acetyllactosame (poly-LacNAc) that was pulled down by wheat germ agglutinin (WGA) and tomato lectin, respectively. Interestingly, the mature form of UT-A3 glycan contains significant amounts of sialic acid. We explored the enzymes responsible for directing UT-A3 sialylation. Sialyltransferase ST6GalI, but not ST3GalIV, catabolizes UT-A3 α2,6-sialylation. Activation of protein kinase C (PKC) by PDB treatment promoted UT-A3 glycan sialylation and membrane surface expression. The PKC inhibitor chelerythrine blocks ST6GalI-induced UT-A3 sialylation. Increased sialylation by ST6GalI increased UT-A3 protein stability and urea transport activity. Collectively, our study reveals a novel mechanism of UT-A3 regulation by ST6GalI-mediated sialylation modification that may play an important role in kidney urea reabsorption and the urinary concentrating mechanism.
Keywords: Glycosylation; Protein kinase C; Sialyltransferase; Urea transporter.
Figures





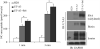
References
-
- Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol. 2007;293:F1308–1313. - PubMed
-
- Bradford AD, Terris JM, Ecelbarger CA, Klein JD, Sands JM, Chou CL, Knepper MA. 97- and 117-kDa forms of collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am J Physiol Renal Physiol. 2001;281:F133–143. - PubMed
-
- Breen KC, Georgopoulou N. The role of protein phosphorylation in alpha2,6(N)-sialyltransferase activity. Biochem Biophys Res Commun. 2003;309:32–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

