Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration
- PMID: 26972991
- PMCID: PMC4793076
- DOI: 10.1038/ncomms10871
Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration
Abstract
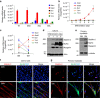
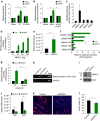
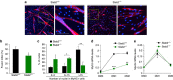
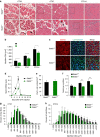
Myoblast fusion is essential for the formation of skeletal muscle myofibres. Studies have shown that phosphatidylserine is necessary for myoblast fusion, but the underlying mechanism is not known. Here we show that the phosphatidylserine receptor stabilin-2 acts as a membrane protein for myoblast fusion during myogenic differentiation and muscle regeneration. Stabilin-2 expression is induced during myogenic differentiation, and is regulated by calcineurin/NFAT signalling in myoblasts. Forced expression of stabilin-2 in myoblasts is associated with increased myotube formation, whereas deficiency of stabilin-2 results in the formation of small, thin myotubes. Stab2-deficient mice have myofibres with small cross-sectional area and few myonuclei and impaired muscle regeneration after injury. Importantly, myoblasts lacking stabilin-2 have reduced phosphatidylserine-dependent fusion. Collectively, our results show that stabilin-2 contributes to phosphatidylserine-dependent myoblast fusion and provide new insights into the molecular mechanism by which phosphatidylserine mediates myoblast fusion during muscle growth and regeneration.
Figures



References
-
- Abmayr S. M., Balagopalan L., Galletta B. J. & Hong S. J. Cell and molecular biology of myoblast fusion. Int. Rev. Cytol. 225, 33–89 (2003). - PubMed
-
- Schlegel R. A. & Williamson P. Phosphatidylserine, a death knell. Cell Death Differ. 8, 551–563 (2001). - PubMed
-
- Fadeel B. & Xue D. PS externalization: from corpse clearance to drug delivery. Cell Death Differ. 13, 360–362 (2006). - PubMed
-
- Van den Eijnde S. M., Boshart L., Reutelingsperger C. P., De Zeeuw C. I. & Vermeij-Keers C. Phosphatidylserine plasma membrane asymmetry in vivo: a pancellular phenomenon which alters during apoptosis. Cell Death Differ. 4, 311–316 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

