Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial
- PMID: 26973324
- PMCID: PMC5063047
- DOI: 10.1016/S1470-2045(15)00614-2
Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial
Abstract
Background: ALK-rearranged non-small-cell lung cancer (NSCLC) is sensitive to ALK tyrosine kinase inhibitors (ALK inhibitors) such as crizotinib, but resistance invariably develops, often with progression in the brain. Ceritinib is a more potent ALK inhibitor than crizotinib in vitro, crosses the blood-brain barrier in vivo, and shows clinical responses in patients with crizotinib-resistant disease. We aimed to assess whole-body activity of ceritinib in both ALK inhibitor-pretreated and ALK inhibitor-naive patients with ALK-rearranged NSCLC.
Methods: ASCEND-1 was an open-label, phase 1 trial that recruited patients from 20 academic hospitals or cancer centres in 11 countries in Europe, North America, and Asia-Pacific. Eligible patients were aged 18 years or older with ALK-rearranged locally advanced or metastatic cancer that had progressed despite standard therapy (or for which no effective standard therapy existed), who had at least one measurable lesion at baseline. The primary objective (to determine the maximum tolerated dose) has been reported previously. This updated analysis includes all patients with ALK-rearranged NSCLC given oral ceritinib at the recommended dose of 750 mg/day in the dose-escalation and expansion phases. Here we report the secondary outcomes of overall response, duration of response, and progression-free survival, analysed in all patients who received at least one 750 mg dose of ceritinib. Exploratory analyses included retrospective analysis of intracranial activity by independent neuroradiologists, in patients with untreated or locally treated neurologically stable brain metastases at baseline. Safety was assessed in all patients who received at least one dose of ceritinib. This study is no longer recruiting patients; however, treatment and follow-up are ongoing. This study is registered with ClinicalTrials.gov, number NCT01283516.
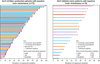
Findings: Between Jan 24, 2011, and July 31, 2013, 255 patients were enrolled and received at least one dose of ceritinib 750 mg/day, of whom 246 had ALK-rearranged NSCLC. At data cutoff (April 14, 2014), median follow-up was 11·1 months (IQR 6·7-15·2) and 147 (60%) patients had discontinued treatment, 98 (40%) as a result of disease progression. An overall response was reported in 60 (72% [95% CI 61-82]) of 83 ALK inhibitor-naive patients and 92 (56% [49-64]) of 163 ALK inhibitor-pretreated patients. Median duration of response was 17·0 months (95% CI 11·3-non-estimable [NE]) in ALK inhibitor-naive patients and 8·3 months (6·8-9·7) in ALK inhibitor-pretreated patients. Median progression-free survival was 18·4 months (95% CI 11·1-NE) in ALK inhibitor-naive patients and 6·9 months (5·6-8·7) in ALK inhibitor-pretreated patients. Of 94 patients with retrospectively confirmed brain metastases and at least one post-baseline MRI or CT tumour assessment, intracranial disease control was reported in 15 (79% [95% CI 54-94]) of 19 ALK inhibitor-naive patients and in 49 (65% [54-76]) of 75 ALK inhibitor-pretreated patients. Of these 94 patients, 11 had measurable brain lesions and no previous radiotherapy to the brain, six of whom achieved a partial intracranial response. Serious adverse events were recorded in 117 (48%) of 246 patients. The most common grade 3-4 laboratory abnormalities were increased alanine aminotransferase (73 [30%] patients) and increased aspartate aminotransferase (25 [10%]). The most common grade 3-4 non-laboratory adverse events were diarrhoea and nausea, both of which occurred in 15 (6%) patients. Two on-treatment deaths during the study were deemed to be related to study drug by the investigators, one due to interstitial lung disease and one as a result of multiorgan failure that occurred in the context of infection and ischaemic hepatitis.
Interpretation: The durable whole-body responses reported, together with the intracranial activity, support a clinical benefit for treatment with ceritinib in patients with ALK-rearranged NSCLC who have received crizotinib, or as an alternative to crizotinib. A confirmatory phase 2 clinical trial is ongoing to assess ceritinib activity in patients with ALK-rearranged NSCLC and brain or leptomeningeal metastases.
Funding: Novartis Pharmaceuticals Corporation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures





Comment in
-
ALK inhibitors: plateauing systemic and intracranial activity?Lancet Oncol. 2016 Apr;17(4):404-406. doi: 10.1016/S1470-2045(16)00025-5. Epub 2016 Mar 11. Lancet Oncol. 2016. PMID: 26973321 No abstract available.
-
Ceritinib in second and further lines of therapy in advanced ALK mutant adenocarcinoma.J Thorac Dis. 2016 Jul;8(7):E584-5. doi: 10.21037/jtd.2016.05.53. J Thorac Dis. 2016. PMID: 27500442 Free PMC article. No abstract available.
-
Second-generation anaplastic lymphoma kinase inhibitors: revolutionary or evolutionary?J Thorac Dis. 2016 Jul;8(7):1425-7. doi: 10.21037/jtd.2016.05.26. J Thorac Dis. 2016. PMID: 27501299 Free PMC article. No abstract available.
References
-
- Chustecka Z. [accessed 20 May 2015];Ceritinib (Zykadia) Recommended for Approval in EU. 2015. 2015 http://www.medscape.com/viewarticle/840585.
-
- Malik SM, Maher VE, Bijwaard KE, et al. U.S. Food and Drug Administration approval: Crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. 2014 - PubMed
-
- [accessed 16 July 2014];Administration UFaD. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm395386.htm.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

