Nonproliferative and Proliferative Lesions of the Gastrointestinal Tract, Pancreas and Salivary Glands of the Rat and Mouse
- PMID: 26973378
- PMCID: PMC4765498
- DOI: 10.1293/tox.29.1S
Nonproliferative and Proliferative Lesions of the Gastrointestinal Tract, Pancreas and Salivary Glands of the Rat and Mouse
Abstract
The INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) project is a joint initiative of the Societies of Toxicologic Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP), and North America (STP) to develop an internationally accepted nomenclature and diagnostic criteria for nonproliferative and proliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature and diagnostic criteria for classifying lesions in the digestive system including the salivary glands and the exocrine pancreas of laboratory rats and mice. Most lesions are illustrated by color photomicrographs. The standardized nomenclature, the diagnostic criteria, and the photomicrographs are also available electronically on the Internet (http://www.goreni.org/). Sources of material included histopathology databases from government, academia, and industrial laboratories throughout the world. Content includes spontaneous and age related lesions as well as lesions induced by exposure to test items. Relevant infectious and parasitic lesions are included as well. A widely accepted and utilized international harmonization of nomenclature and diagnostic criteria for the digestive system will decrease misunderstandings among regulatory and scientific research organizations in different countries and provide a common language to increase and enrich international exchanges of information among toxicologists and pathologists.
Keywords: diagnostic criteria; diagnostic pathology; digestive system or tract; esophagus; intestine; large intestine; nomenclature; oral cavity; pancreas se; salivary glands; small intestine; stomach.
Figures

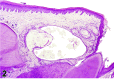
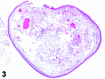


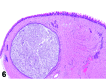

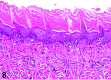
































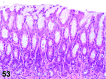














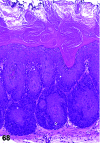
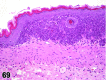

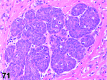



















































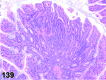
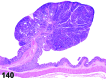
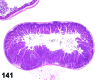





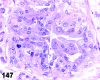









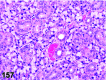
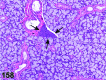
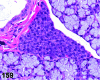
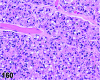








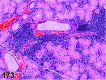
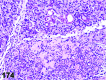

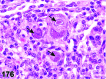
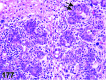


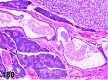
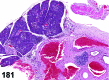
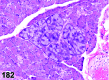

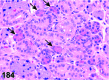
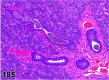
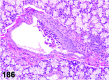




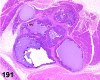
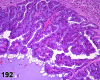
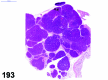



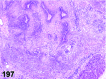

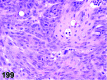



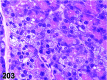









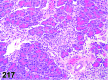







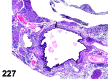



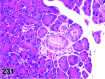



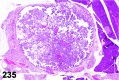
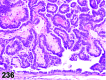

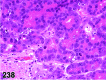

References
-
- Abe K, and Watanabe S. Apoptosis of mouse pancreatic acinar cells after duct ligation. Arch Histol Cytol. 58: 221–229. 1995. - PubMed
-
- Aguirre SA, Liu L, Hosea NA, Scott W, May JR, Burns-Naas LA, Randolph S, Denlinger RH, and Han B. Intermittent oral coadministration of a gamma secretase inhibitor with dexamethasone mitigates intestinal goblet cell hyperplasia in rats. Toxicol Pathol. 42: 422–434. 2014. - PubMed
-
- Anderson LC. Salivary gland structure and function in experimental diabetes mellitus. Biomedical Reviews. 9: 107–119. 1998.
-
- Andersson P, Rubio C, Poellinger L, and Hanberg A. Gastric hamartomatous tumours in a transgenic mouse model expressing an activated dioxin/Ah receptor. Anticancer Res. 25(2A): 903–911. 2005. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
