De novo autoimmune hepatitis in liver transplant: State-of-the-art review
- PMID: 26973387
- PMCID: PMC4779914
- DOI: 10.3748/wjg.v22.i10.2906
De novo autoimmune hepatitis in liver transplant: State-of-the-art review
Abstract
In the two past decades, a number of communications, case-control studies, and retrospective reports have appeared in the literature with concerns about the development of a complex set of clinical, laboratory and histological characteristics of a liver graft dysfunction that is compatible with autoimmune hepatitis. The de novo prefix was added to distinguish this entity from a pre-transplant primary autoimmune hepatitis, but the globally accepted criteria for the diagnosis of autoimmune hepatitis have been adopted in the diagnostic algorithm. Indeed, de novo autoimmune hepatitis is characterized by the typical liver necro-inflammation that is rich in plasma cells, the presence of interface hepatitis and the consequent laboratory findings of elevations in liver enzymes, increases in serum gamma globulin and the appearance of non-organ specific auto-antibodies. Still, the overall features of de novo autoimmune hepatitis appear not to be attributable to a univocal patho-physiological pathway because they can develop in the patients who have undergone liver transplantation due to different etiologies. Specifically, in subjects with hepatitis C virus recurrence, an interferon-containing antiviral treatment has been indicated as a potential inception of immune system derangement. Herein, we attempt to review the currently available knowledge about de novo liver autoimmunity and its clinical management.
Keywords: Antiviral therapy; Autoimmunity; De novo autoimmune hepatitis; Differential diagnosis; Hepatitis C virus recurrence; Liver transplant; Plasma-cell hepatitis.
Figures
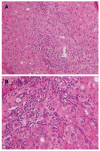
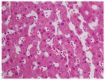
References
-
- Hübscher SG. What is the long-term outcome of the liver allograft? J Hepatol. 2011;55:702–717. - PubMed
-
- Selzner N, Guindi M, Renner EL, Berenguer M. Immune-mediated complications of the graft in interferon-treated hepatitis C positive liver transplant recipients. J Hepatol. 2011;55:207–217. - PubMed
-
- Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3–26. - PubMed
-
- Fiel MI, Agarwal K, Stanca C, Elhajj N, Kontorinis N, Thung SN, Schiano TD. Posttransplant plasma cell hepatitis (de novo autoimmune hepatitis) is a variant of rejection and may lead to a negative outcome in patients with hepatitis C virus. Liver Transpl. 2008;14:861–871. - PubMed
-
- Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12:1813–1824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

