Titin, a Central Mediator for Hypertrophic Signaling, Exercise-Induced Mechanosignaling and Skeletal Muscle Remodeling
- PMID: 26973541
- PMCID: PMC4771757
- DOI: 10.3389/fphys.2016.00076
Titin, a Central Mediator for Hypertrophic Signaling, Exercise-Induced Mechanosignaling and Skeletal Muscle Remodeling
Abstract
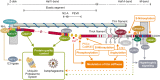
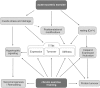
Titin is a giant scaffold protein with multiple functions in striated muscle physiology. Due to the elastic I-band domains and the filament-like integration in the half-sarcomere titin is an important factor for sarcomere assembly and serves as an adaptable molecular spring that determines myofilament distensibility. Protein-interactions e.g., with muscle ankyrin repeat proteins or muscle LIM-protein link titin to hypertrophic signaling and via p62 and Muscle Ring Finger proteins to mechanisms that control protein quality control. This review summarizes our current knowledge on titin as a central node for exercise-induced mechanosignaling and remodeling and further highlights the pathophysiological implications.
Keywords: connection; hypertrophic signaling; passive tension; posttranslational modification; striated muscle.
Figures
References
-
- Bang M. L., Centner T., Fornoff F., Geach A. J., Gotthardt M., Mc- Nabb M., et al. . (2001). The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ. Res. 89, 1065–1072. 10.1161/hh2301.100981 - DOI - PubMed
-
- Bang M. L., Gu Y., Dalton N. D., Peterson K. L., Chien K. R., Chen J. (2014). The muscle ankyrin repeat proteins CARP, Ankrd2, and DARP are not essential for normal cardiac development and function at basal conditions and in response to pressure overload. PLoS ONE 9:e93638 10.1371/journal.pone.0093638 - DOI - PMC - PubMed
-
- Bartoo M. L., Linke W. A., and Pollack G. H. (1997). Basis of passive tension and stiffness in isolated rabbit myofibrils. Am. J. Physiol. Cell. Physiol. 273, C266–C276. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

