Reconstructing the Origin of Oxygenic Photosynthesis: Do Assembly and Photoactivation Recapitulate Evolution?
- PMID: 26973693
- PMCID: PMC4773611
- DOI: 10.3389/fpls.2016.00257
Reconstructing the Origin of Oxygenic Photosynthesis: Do Assembly and Photoactivation Recapitulate Evolution?
Abstract
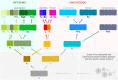
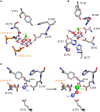
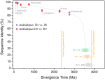
Due to the great abundance of genomes and protein structures that today span a broad diversity of organisms, now more than ever before, it is possible to reconstruct the molecular evolution of protein complexes at an incredible level of detail. Here, I recount the story of oxygenic photosynthesis or how an ancestral reaction center was transformed into a sophisticated photochemical machine capable of water oxidation. First, I review the evolution of all reaction center proteins in order to highlight that Photosystem II and Photosystem I, today only found in the phylum Cyanobacteria, branched out very early in the history of photosynthesis. Therefore, it is very unlikely that they were acquired via horizontal gene transfer from any of the described phyla of anoxygenic phototrophic bacteria. Second, I present a new evolutionary scenario for the origin of the CP43 and CP47 antenna of Photosystem II. I suggest that the antenna proteins originated from the remodeling of an entire Type I reaction center protein and not from the partial gene duplication of a Type I reaction center gene. Third, I highlight how Photosystem II and Photosystem I reaction center proteins interact with small peripheral subunits in remarkably similar patterns and hypothesize that some of this complexity may be traced back to the most ancestral reaction center. Fourth, I outline the sequence of events that led to the origin of the Mn4CaO5 cluster and show that the most ancestral Type II reaction center had some of the basic structural components that would become essential in the coordination of the water-oxidizing complex. Finally, I collect all these ideas, starting at the origin of the first reaction center proteins and ending with the emergence of the water-oxidizing cluster, to hypothesize that the complex and well-organized process of assembly and photoactivation of Photosystem II recapitulate evolutionary transitions in the path to oxygenic photosynthesis.
Keywords: anoxygenic photosynthesis; oxygenic photosynthesis; photoactivation; photoassembly; photosystem; reaction center; water oxidation.
Figures



References
-
- Allen J. F., Vermaas W. (2010). Evolution of photosynthesis, in Encyclopedia of Life Sciences (els), (Chichester: John Wiley & Sons, Ltd; ). Available online at: http://jfallen.org/publications/pdf/Allen_2010_ELS.pdf
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

