Randomized double-blind safety comparison of intravenous iron dextran versus iron sucrose in an adult non-hemodialysis outpatient population: A feasibility study
- PMID: 26973791
- PMCID: PMC4788943
- DOI: 10.1186/s12878-016-0046-8
Randomized double-blind safety comparison of intravenous iron dextran versus iron sucrose in an adult non-hemodialysis outpatient population: A feasibility study
Abstract
Background: Intravenous iron therapy is a treatment option for iron deficient patients who are intolerant to oral iron or where oral iron is ineffective, but with possible adverse effects. Currently, prospective studies comparing different intravenous iron formulations are needed to determine safety and efficacy of these agents.
Methods: We conducted a prospective, double-blind, randomized controlled trial (RCT) to assess the feasibility of a trial comparing the safety of high molecular weight intravenous iron dextran, Infufer®, with intravenous iron sucrose, Venofer®, in non-hemodialysis adult outpatients. Primary outcome was the occurrence of immediate severe drug reactions.
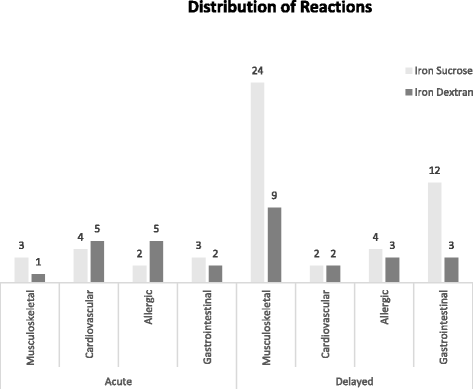
Results: We enrolled 143 patients in a one-year period. Overall, 45/143 (31.5 %) patients (20 iron dextran, 25 iron sucrose) developed 48 infusion reactions (14 immediate, 28 delayed, and 3 both). The risk of an immediate reaction was similar in both groups, 9/73 (12.3 %) iron dextran versus 8/70 (11.4 %) iron sucrose, RR = 0.93 (95 % CI; 0.38 to 2.27). The risk of a delayed reaction was significantly higher in the iron sucrose group 22/70 (31.4 %) versus the iron dextran group 9/73 (12.3 %), RR = 2.55 (95 % CI; 1.26 to 5.15; p = 0.0078).
Conclusion: In this limited feasibility study, no major differences in immediate reactions were seen, but a significantly higher number of delayed reactions were seen in the iron sucrose group. Further, under our assumptions and design a full RCT to evaluate the safety of different intravenous iron preparations is not feasible. Future studies should consider modifying the clinical outcomes, utilize multiple centers, and consider other emerging parenteral iron formulations. (ClinicalTrials.gov NCT005936197 January 3, 2008).
Keywords: Feasibility; Intravenous iron; Iron dextran; Iron sucrose; Randomized controlled trial; Safety.
Figures
Similar articles
-
A Comparative Look at the Safety Profiles of Intravenous Iron Products Used in the Hemodialysis Population.Ann Pharmacother. 2011 Feb;45(2):241-7. doi: 10.1345/aph.1P466. Ann Pharmacother. 2011. PMID: 21304025 Review.
-
Comparison of intravenous low molecular weight iron dextran and intravenous iron sucrose for the correction of anaemia in pre-dialysis chronic kidney disease patients: a randomized single-centre study in Nigeria.Clin Kidney J. 2016 Dec;9(6):817-822. doi: 10.1093/ckj/sfw064. Epub 2016 Jul 21. Clin Kidney J. 2016. PMID: 27994861 Free PMC article.
-
Comparison of intravenous iron sucrose versus low-molecular-weight iron dextran in chronic kidney disease.J Ren Care. 2009 Jun;35(2):67-73. doi: 10.1111/j.1755-6686.2009.00099.x. J Ren Care. 2009. PMID: 19432851
-
Sodium ferric gluconate complex in hemodialysis patients. II. Adverse reactions in iron dextran-sensitive and dextran-tolerant patients.Kidney Int. 2003 Jan;63(1):217-24. doi: 10.1046/j.1523-1755.2003.00703.x. Kidney Int. 2003. PMID: 12472786 Clinical Trial.
-
The available intravenous iron formulations: History, efficacy, and toxicology.Hemodial Int. 2017 Jun;21 Suppl 1:S83-S92. doi: 10.1111/hdi.12560. Epub 2017 Mar 29. Hemodial Int. 2017. PMID: 28371203 Review.
Cited by
-
How Do Cyclodextrins and Dextrans Affect the Gut Microbiome? Review of Prebiotic Activity.Molecules. 2024 Nov 11;29(22):5316. doi: 10.3390/molecules29225316. Molecules. 2024. PMID: 39598705 Free PMC article. Review.
-
Intravenous iron therapy for non-anaemic, iron-deficient adults.Cochrane Database Syst Rev. 2019 Dec 20;12(12):CD013084. doi: 10.1002/14651858.CD013084.pub2. Cochrane Database Syst Rev. 2019. PMID: 31860749 Free PMC article.
-
Decreased Clinical Toxicity and Two-Phase Elimination Kinetics Observed After Intravenous Iron Sucrose Overdose.J Emerg Med. 2022 Dec;63(6):766-771. doi: 10.1016/j.jemermed.2022.09.005. Epub 2022 Oct 19. J Emerg Med. 2022. PMID: 36270861 Free PMC article.
-
Acute aortic intraluminal thrombus with embolisation and lower-limb ischaemia following intravenous iron sucrose infusion reaction.BMJ Case Rep. 2023 Sep 27;16(9):e255702. doi: 10.1136/bcr-2023-255702. BMJ Case Rep. 2023. PMID: 37758660 Free PMC article.
References
-
- 2014 Annual Results Report: Nutrition. New York: UNICEF; 2015. p. 23. http://www.unicef.org/publicpartnerships/files/2014_Annual_Results_Repor.... Accessed 10 March 2016.
LinkOut - more resources
Full Text Sources
Other Literature Sources