The Pathogen-Occupied Vacuoles of Anaplasma phagocytophilum and Anaplasma marginale Interact with the Endoplasmic Reticulum
- PMID: 26973816
- PMCID: PMC4771727
- DOI: 10.3389/fcimb.2016.00022
The Pathogen-Occupied Vacuoles of Anaplasma phagocytophilum and Anaplasma marginale Interact with the Endoplasmic Reticulum
Abstract
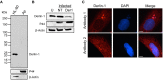
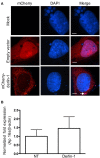
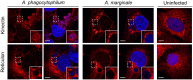
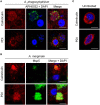
The genus Anaplasma consists of tick-transmitted obligate intracellular bacteria that invade white or red blood cells to cause debilitating and potentially fatal infections. A. phagocytophilum, a human and veterinary pathogen, infects neutrophils to cause granulocytic anaplasmosis. A. marginale invades bovine erythrocytes. Evidence suggests that both species may also infect endothelial cells in vivo. In mammalian and arthropod host cells, A. phagocytophilum and A. marginale reside in host cell derived pathogen-occupied vacuoles (POVs). While it was recently demonstrated that the A. phagocytophilum-occupied vacuole (ApV) intercepts membrane traffic from the trans-Golgi network, it is unclear if it or the A. marginale-occupied vacuole (AmV) interacts with other secretory organelles. Here, we demonstrate that the ApV and AmV extensively interact with the host endoplasmic reticulum (ER) in endothelial, myeloid, and/or tick cells. ER lumen markers, calreticulin, and protein disulfide isomerase, and the ER membrane marker, derlin-1, were pronouncedly recruited to the peripheries of both POVs. ApV association with the ER initiated early and continued throughout the infection cycle. Both the ApV and AmV interacted with the rough ER and smooth ER. However, only derlin-1-positive rough ER derived vesicles were delivered into the ApV lumen where they localized with intravacuolar bacteria. Transmission electron microscopy identified multiple ER-POV membrane contact sites on the cytosolic faces of both species' vacuoles that corresponded to areas on the vacuoles' lumenal faces where intravacuolar Anaplasma organisms closely associated. A. phagocytophilum is known to hijack Rab10, a GTPase that regulates ER dynamics and morphology. Yet, ApV-ER interactions were unhindered in cells in which Rab10 had been knocked down, demonstrating that the GTPase is dispensable for the bacterium to parasitize the ER. These data establish the ApV and AmV as pathogen-host interfaces that directly engage the ER in vertebrate and invertebrate host cells and evidence the conservation of ER parasitism between two Anaplasma species.
Keywords: Anaplasmataceae; Rab; Rickettsia; endoplasmic reticulum; intracellular bacteria; pathogen synapse.
Figures







Similar articles
-
The Anaplasma phagocytophilum-occupied vacuole selectively recruits Rab-GTPases that are predominantly associated with recycling endosomes.Cell Microbiol. 2010 Sep 1;12(9):1292-307. doi: 10.1111/j.1462-5822.2010.01468.x. Epub 2010 Mar 25. Cell Microbiol. 2010. PMID: 20345488 Free PMC article.
-
Anaplasma phagocytophilum APH0032 Is Exposed on the Cytosolic Face of the Pathogen-Occupied Vacuole and Co-opts Host Cell SUMOylation.Front Cell Infect Microbiol. 2016 Sep 22;6:108. doi: 10.3389/fcimb.2016.00108. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27713867 Free PMC article.
-
Anaplasma phagocytophilum Rab10-dependent parasitism of the trans-Golgi network is critical for completion of the infection cycle.Cell Microbiol. 2016 Feb;18(2):260-81. doi: 10.1111/cmi.12500. Epub 2015 Oct 7. Cell Microbiol. 2016. PMID: 26289115 Free PMC article.
-
Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance.Parasitol Res. 2015 Nov;114(11):3941-57. doi: 10.1007/s00436-015-4698-2. Epub 2015 Sep 7. Parasitol Res. 2015. PMID: 26346451 Review.
-
Adaptive immunity to Anaplasma pathogens and immune dysregulation: implications for bacterial persistence.Comp Immunol Microbiol Infect Dis. 2012 May;35(3):241-52. doi: 10.1016/j.cimid.2011.12.002. Epub 2012 Jan 4. Comp Immunol Microbiol Infect Dis. 2012. PMID: 22226382 Free PMC article. Review.
Cited by
-
The Prostaglandin E2-EP3 Receptor Axis Regulates Anaplasma phagocytophilum-Mediated NLRC4 Inflammasome Activation.PLoS Pathog. 2016 Aug 2;12(8):e1005803. doi: 10.1371/journal.ppat.1005803. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27482714 Free PMC article.
-
Rickettsial pathogen inhibits tick cell death through tryptophan metabolite mediated activation of p38 MAP kinase.iScience. 2022 Dec 5;26(1):105730. doi: 10.1016/j.isci.2022.105730. eCollection 2023 Jan 20. iScience. 2022. PMID: 36582833 Free PMC article.
-
The Obligate Intracellular Bacterial Pathogen Anaplasma phagocytophilum Exploits Host Cell Multivesicular Body Biogenesis for Proliferation and Dissemination.mBio. 2022 Dec 20;13(6):e0296122. doi: 10.1128/mbio.02961-22. Epub 2022 Nov 21. mBio. 2022. PMID: 36409075 Free PMC article.
-
Binding of Host Cell Surface Protein Disulfide Isomerase by Anaplasma phagocytophilum Asp14 Enables Pathogen Infection.mBio. 2020 Jan 28;11(1):e03141-19. doi: 10.1128/mBio.03141-19. mBio. 2020. PMID: 31992623 Free PMC article.
-
The Novel Zoonotic Pathogen, Anaplasma capra, Infects Human Erythrocytes, HL-60, and TF-1 Cells In Vitro.Pathogens. 2021 May 14;10(5):600. doi: 10.3390/pathogens10050600. Pathogens. 2021. PMID: 34069112 Free PMC article.
References
-
- Agaisse H., Derre I. (2014). Expression of the effector protein IncD in Chlamydia trachomatis mediates recruitment of the lipid transfer protein CERT and the endoplasmic reticulum-resident protein VAPB to the inclusion membrane. Infect. Immun. 82, 2037–2047. 10.1128/IAI.01530-14 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

