Mitigation of Ergot Vasoconstriction by Clover Isoflavones in Goats (Capra hircus)
- PMID: 26973844
- PMCID: PMC4777723
- DOI: 10.3389/fvets.2016.00017
Mitigation of Ergot Vasoconstriction by Clover Isoflavones in Goats (Capra hircus)
Abstract
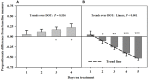
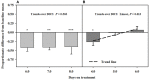
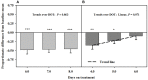
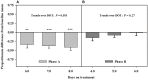
Ergot alkaloids produced by a fungal endophyte (Epichloë coenophiala; formerly Neotyphodium coenophialum) that infects tall fescue (Lolium arundinaceum) can induce persistent constriction of the vasculature in ruminants, hindering their capability to thermo-regulate core body temperature. There is evidence that isoflavones produced by legumes can relax the vasculature, which suggests that they could relieve ergot alkaloid-induced vasoconstriction and mitigate the vulnerability to severe heat stress in ruminants that graze tall fescue. To test if isoflavones can relieve alkaloid-induced vasoconstriction, two pen experiments were conducted with rumen-fistulated goats (Capra hircus) to determine with ultrasonograpy if isoflavones can (1) promote vascular compliance by countering alkaloid-induced vasoconstriction and (2) relieve already imposed alkaloid-induced vasoconstriction. Goats were fed ad libitum chopped orchardgrass (Dactylis glomerata)-timothy (Phleum pratense) hay prior to conducting the experiments. Measures of carotid and interosseous luminal areas were obtained pre- (baseline) and post-ruminal infusions in both experiments with goats being fed the hay, and for blood flow rate in the carotid artery in Experiment 2. Responses to infusion treatments were evaluated as proportionate differences from baseline measures. Peak systolic velocity, pulsatility index, and heart rate were measured on the last day on treatment (DOT) in Experiment 1, and on all imaging sessions during Experiment 2. For Experiment 1, rumens were infused with ground toxic fescue seed and isoflavones in Phase A and with only the toxic seed in Phase B. The infusion treatments were switched between phases in Experiment 2, which employed a fescue seed extract having an ergot alkaloid composition equivalent to that of the ground seed used in Experiment 1. During Experiment 1, luminal areas of carotid and interosseous arteries in Phase A did not deviate (P > 0.1) from baselines over 1, 2, 3, and 4 DOT, but the areas of both declined linearly from baselines over 1, 2, 3, and 4 DOT in Phase B. By 6, 7, and 8 DOT in Experiment 2, luminal areas of the arteries and flow rate declined from baselines with infusions with the only seed extract in Phase A, but luminal areas and flow rate increased over 4, 5, and 6 DOT with the additional infusion of isoflavones. Peak systolic velocity and heart rate were not affected by treatment in either experiment, but were highest when infused with only ergot alkaloids in both experiments. Treatment with isoflavones was demonstrated to relax the carotid and interosseous arteries and reduce resistance to blood flow. Results indicate that isoflavones can relax persistent vasoconstriction in goats caused by consumption of ergot alkaloids, and mitigate the adverse effect that ergot alkaloids have on dry matter intake.
Keywords: ergot alkaloids; goats; isoflavones; tall fescue; vasoconstriction.
Figures

Similar articles
-
Isoflavone Containing Legumes Mitigate Ergot Alkaloid-Induced Vasoconstriction in Goats (Capra hircus).Animals (Basel). 2022 Mar 17;12(6):750. doi: 10.3390/ani12060750. Animals (Basel). 2022. PMID: 35327147 Free PMC article.
-
Vasoconstrictive responses by the carotid and auricular arteries in goats to ergot alkaloid exposure.Front Chem. 2014 Nov 20;2:101. doi: 10.3389/fchem.2014.00101. eCollection 2014. Front Chem. 2014. PMID: 25478559 Free PMC article.
-
Vasoactivity and Vasoconstriction Changes in Cattle Related to Time off Toxic Endophyte-Infected Tall Fescue.Toxins (Basel). 2016 Sep 22;8(10):271. doi: 10.3390/toxins8100271. Toxins (Basel). 2016. PMID: 27669299 Free PMC article.
-
Forages and pastures symposium: managing the tall fescue-fungal endophyte symbiosis for optimum forage-animal production.J Anim Sci. 2013 May;91(5):2369-78. doi: 10.2527/jas.2012-5948. Epub 2013 Jan 10. J Anim Sci. 2013. PMID: 23307847 Review.
-
Ergot alkaloid intoxication in perennial ryegrass (Lolium perenne): an emerging animal health concern in Ireland?Ir Vet J. 2014 Sep 25;67(1):21. doi: 10.1186/2046-0481-67-21. eCollection 2014. Ir Vet J. 2014. PMID: 25295161 Free PMC article. Review.
Cited by
-
Rumen and Serum Metabolomes in Response to Endophyte-Infected Tall Fescue Seed and Isoflavone Supplementation in Beef Steers.Toxins (Basel). 2020 Nov 26;12(12):744. doi: 10.3390/toxins12120744. Toxins (Basel). 2020. PMID: 33256042 Free PMC article.
-
Red clover supplementation modifies rumen fermentation and promotes feed efficiency in ram lambs.J Anim Sci. 2023 Jan 3;101:skad036. doi: 10.1093/jas/skad036. J Anim Sci. 2023. PMID: 36751104 Free PMC article.
-
Effects of endophyte-infected tall fescue seed and red clover isoflavones on rumen microbial populations and physiological parameters of beef cattle.Transl Anim Sci. 2018 Dec 29;3(1):315-328. doi: 10.1093/tas/txy147. eCollection 2019 Jan. Transl Anim Sci. 2018. PMID: 32704802 Free PMC article.
-
Performance-enhancing technologies for steers grazing tall fescue pastures with varying levels of toxicity1.J Anim Sci. 2018 Sep 7;96(9):3712-3727. doi: 10.1093/jas/sky244. J Anim Sci. 2018. PMID: 29917102 Free PMC article.
-
Isoflavone Containing Legumes Mitigate Ergot Alkaloid-Induced Vasoconstriction in Goats (Capra hircus).Animals (Basel). 2022 Mar 17;12(6):750. doi: 10.3390/ani12060750. Animals (Basel). 2022. PMID: 35327147 Free PMC article.
References
-
- Sleper DA, West CP. Tall fescue. In: Moser LE, Buxton DR, Casler MD, editors. Cool-Season Grass Forages. Madison, WI: ASA, CSSA, and SSSA Agronomy Monograph No. 34; (1996). p. 471–505.
-
- Schmidt SP, Osborn TG. Effects of endophyte-infected tall fescue on animal performance. Agric Ecosyst Environ (1993) 44:233–62.10.1016/0167-8809(93)90049-U - DOI
-
- Oliver J. Pathophysiologic response to endophyte toxins. In: Roberts CA, West CP, Spiers DE, editors. Neotyphodium in Cool-Season Grasses. Ames, IA: Blackwell Publishing; (2005). p. 291–304.
-
- Rhodes MT, Paterson JA, Kerley MS, Garner HE, Laughlin MH. Reduced blood flow to peripheral and core body temperatures in sheep and cattle induced by endophyte-infected tall fescue. J Anim Sci (1991) 69:2033–43. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

