Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci
- PMID: 26974007
- PMCID: PMC4848113
- DOI: 10.1038/ng.3528
Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci
Abstract
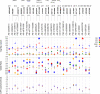
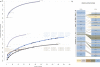
We simultaneously investigated the genetic landscape of ankylosing spondylitis, Crohn's disease, psoriasis, primary sclerosing cholangitis and ulcerative colitis to investigate pleiotropy and the relationship between these clinically related diseases. Using high-density genotype data from more than 86,000 individuals of European ancestry, we identified 244 independent multidisease signals, including 27 new genome-wide significant susceptibility loci and 3 unreported shared risk loci. Complex pleiotropy was supported when contrasting multidisease signals with expression data sets from human, rat and mouse together with epigenetic and expressed enhancer profiles. The comorbidities among the five immune diseases were best explained by biological pleiotropy rather than heterogeneity (a subgroup of cases genetically identical to those with another disease, possibly owing to diagnostic misclassification, molecular subtypes or excessive comorbidity). In particular, the strong comorbidity between primary sclerosing cholangitis and inflammatory bowel disease is likely the result of a unique disease, which is genetically distinct from classical inflammatory bowel disease phenotypes.
Figures

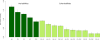
Comment in
-
Genetics: Novel insights from large cross-disease study.Nat Rev Rheumatol. 2016 May;12(5):253. doi: 10.1038/nrrheum.2016.50. Epub 2016 Apr 4. Nat Rev Rheumatol. 2016. PMID: 27044759 No abstract available.
References
-
- Parkes M, Cortes A, van Heel DA, Brown MA. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat Rev Genet. 2013;14:661–73. - PubMed
-
- Najarian DJ, Gottlieb AB. Connections between psoriasis and Crohn's disease. J Am Acad Dermatol. 2003;48:805–21. quiz 822-4. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AR042742/AR/NIAMS NIH HHS/United States
- R01 DK084960/DK/NIDDK NIH HHS/United States
- C0482/MRF_/MRF_/United Kingdom
- R01 AR063759/AR/NIAMS NIH HHS/United States
- UH2 AR067677/AR/NIAMS NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- G0800675/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
- U19 AI111224/AI/NIAID NIH HHS/United States
- U01 GM092691/GM/NIGMS NIH HHS/United States
- R01 AR050511/AR/NIAMS NIH HHS/United States
- R01 AR065183/AR/NIAMS NIH HHS/United States
- R01 AR063611/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

