Effect of Providing Multiple Micronutrients in Powder through Primary Healthcare on Anemia in Young Brazilian Children: A Multicentre Pragmatic Controlled Trial
- PMID: 26974146
- PMCID: PMC4790963
- DOI: 10.1371/journal.pone.0151097
Effect of Providing Multiple Micronutrients in Powder through Primary Healthcare on Anemia in Young Brazilian Children: A Multicentre Pragmatic Controlled Trial
Erratum in
-
Correction: Effect of Providing Multiple Micronutrients in Powder through Primary Healthcare on Anemia in Young Brazilian Children: A Multicentre Pragmatic Controlled Trial.PLoS One. 2016 May 18;11(5):e0156194. doi: 10.1371/journal.pone.0156194. eCollection 2016. PLoS One. 2016. PMID: 27192494 Free PMC article.
Abstract
Background: Multiple micronutrients in powder (MNP) are recommended by WHO to prevent anemia in young children. However, evidences for its effectiveness in different populations and improvements in other outcomes (e.g. linear growth and vitamin A deficiency) are scarce.
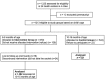
Methods: A multicentre pragmatic controlled trial was carried out in primary health centres. At study baseline, a control group (CG) of children aged 10- to 14 months (n = 521) was recruited in the routine healthcare for assessing anemia, anthropometric and micronutrient status. At the same time, an intervention group (IG) of infants aged 6- to 8 months (n = 462) was recruited to receive MNP daily in complementary feeding over a period of 60 days. Both study groups were compared when the IG infants reached the age of the CG children at enrolment.
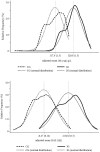
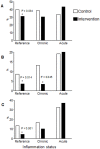
Results: In CG, the prevalence of anemia [hemoglobin (Hb) < 110 g/L], iron deficiency (ID, plasma ferritin < 12 μg/L or TfR > 8.3 mg/L), and vitamin A deficiency (VAD, serum retinol < 0.70μmol/L) were 23.1%, 37.4%, and 17.4%, respectively. Four to six months after enrolment, when the IG participants had the same age of the controls at the time of testing, the prevalence of anemia, ID and VAD in IG were 14.3%, 30.1% and 7.9%, respectively. Adjusting for city, health centre, maternal education, and age, IG children had a lower likelihood of anemia and VAD [Prevalence Ratio (95% CI) = 0.63 (0.45, 0.88) and 0.45 (0.29, 0.69), respectively] when compared with CG children. The adjusted mean distributions of Hb and length-for-age Z-scores improved by 2 SE in the IG compared to CG children.
Conclusions: MNP effectively reduced anemia and improved growth and micronutrient status among young Brazilian children.
Trial registration: Registro Brasileiro de Ensaios Clinicos RBR-5ktv6b.
Conflict of interest statement
Figures
Similar articles
-
Multiple micronutrients in powder delivered through primary health care reduce iron and vitamin A deficiencies in young Amazonian children.Public Health Nutr. 2016 Nov;19(16):3039-3047. doi: 10.1017/S1368980016001294. Epub 2016 May 30. Public Health Nutr. 2016. PMID: 27237018 Free PMC article. Clinical Trial.
-
The impact of home fortification with multiple micronutrient powder on vitamin A status in young children: A multicenter pragmatic controlled trial in Brazil.Matern Child Nutr. 2017 Oct;13(4):e12403. doi: 10.1111/mcn.12403. Epub 2016 Dec 7. Matern Child Nutr. 2017. PMID: 27925426 Free PMC article. Clinical Trial.
-
An Integrated Infant and Young Child Feeding and Micronutrient Powder Intervention Does Not Affect Anemia, Iron Status, or Vitamin A Status among Children Aged 12-23 Months in Eastern Uganda.J Nutr. 2020 Apr 1;150(4):938-944. doi: 10.1093/jn/nxz314. J Nutr. 2020. PMID: 31923315 Free PMC article.
-
Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review).Evid Based Child Health. 2013 Jan;8(1):112-201. doi: 10.1002/ebch.1895. Evid Based Child Health. 2013. PMID: 23878126 Review.
-
Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age.Cochrane Database Syst Rev. 2020 Feb 28;2(2):CD008959. doi: 10.1002/14651858.CD008959.pub3. Cochrane Database Syst Rev. 2020. PMID: 32107773 Free PMC article.
Cited by
-
Prevalence of multiple micronutrient powders consumption and its determinants among 6- to 23-month-old children in East Africa: a mixed effect analysis using the recent population based cross sectional national health survey.BMC Nutr. 2024 May 31;10(1):79. doi: 10.1186/s40795-024-00888-0. BMC Nutr. 2024. PMID: 38822432 Free PMC article.
-
Fortification of staple foods with vitamin A for vitamin A deficiency.Cochrane Database Syst Rev. 2019 May 10;5(5):CD010068. doi: 10.1002/14651858.CD010068.pub2. Cochrane Database Syst Rev. 2019. PMID: 31074495 Free PMC article.
-
Adherence to and acceptability of home fortification with vitamins and minerals in children aged 6 to 23 months: a systematic review.BMC Public Health. 2016 Apr 7;16:299. doi: 10.1186/s12889-016-2978-0. BMC Public Health. 2016. PMID: 27056182 Free PMC article.
-
Prevalence and correlates of childhood anemia in the MINA-Brazil birth cohort study.Rev Saude Publica. 2024 Feb 26;57Suppl 2(Suppl 2):6s. doi: 10.11606/s1518-8787.2023057005637. eCollection 2024. Rev Saude Publica. 2024. PMID: 38422335 Free PMC article.
-
Micronutrient powder supplements combined with nutrition education marginally improve growth amongst children aged 6-23 months in rural Burkina Faso: A cluster randomized controlled trial.Matern Child Nutr. 2019 Oct;15(4):e12820. doi: 10.1111/mcn.12820. Epub 2019 Jun 10. Matern Child Nutr. 2019. PMID: 30941887 Free PMC article. Clinical Trial.
References
-
- World Health Organization. Center for Disease Control and Prevention. de Benoist B, McLean E, Egli I, Cogswellm M, editors. Worldwide prevalence of anaemia 1993–2005 WHO Global Database on Anaemia. Geneva: WHO, 2008.
-
- World Health Organization. Use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age. Geneva: WHO, 2011. - PubMed
-
- World Health Organization. Iron deficiency anaemia: assessment, prevention and control A guide for programme managers. Geneva: WHO, 2001.
-
- Jordão RE, Bernardi JLD, Barros Filho A. Prevalência de anemia ferropriva no Brasil: uma revisão sistemática (Prevalence of iron-deficiency anemia in Brazil: a systematic review). Rev Paul Pediatr 2009;27:90–8. Portuguese.
-
- Vieira RCS, Ferreira HS. Prevalência de anemia em crianças brasileiras, segundo diferentes cenários epidemiológicos(Prevalence of anemia in Brazilian children indiferente epidemiological scenarios). Rev Nutr 2010;23:433–44. Portuguese.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

