Oxidized Ferric and Ferryl Forms of Hemoglobin Trigger Mitochondrial Dysfunction and Injury in Alveolar Type I Cells
- PMID: 26974230
- PMCID: PMC4979363
- DOI: 10.1165/rcmb.2015-0197OC
Oxidized Ferric and Ferryl Forms of Hemoglobin Trigger Mitochondrial Dysfunction and Injury in Alveolar Type I Cells
Abstract
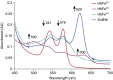
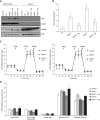
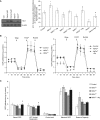
Lung alveoli are lined by alveolar type (AT) 1 cells and cuboidal AT2 cells. The AT1 cells are likely to be exposed to cell-free hemoglobin (Hb) in multiple lung diseases; however, the role of Hb redox (reduction-oxidation) reactions and their precise contributions to AT1 cell injury are not well understood. Using mouse lung epithelial cells (E10) as an AT1 cell model, we demonstrate here that higher Hb oxidation states, ferric Hb (HbFe(3+)) and ferryl Hb (HbFe(4+)) and subsequent heme loss play a central role in the genesis of injury. Exposures to HbFe(2+) and HbFe(3+) for 24 hours induced expression of heme oxygenase (HO)-1 protein in E10 cells and HO-1 translocation in the purified mitochondrial fractions. Both of these effects were intensified with increasing oxidation states of Hb. Next, we examined the effects of Hb oxidation and free heme on mitochondrial bioenergetic function by measuring changes in the mitochondrial transmembrane potential and oxygen consumption rate. In contrast to HbFe(2+), HbFe(3+) reduced basal oxygen consumption rate, indicating compromised mitochondrial activity. However, HbFe(4+) exposure not only induced early expression of HO-1 but also caused mitochondrial dysfunction within 12 hours when compared with HbFe(2+) and HbFe(3+). Exposure to HbFe(4+) for 24 hours also caused mitochondrial depolarization in E10 cells. The deleterious effects of HbFe(3+) and HbFe(4+) were reversed by the addition of scavenger proteins, haptoglobin and hemopexin. Collectively, these data establish, for the first time, a central role for cell-free Hb in lung epithelial injury, and that these effects are mediated through the redox transition of Hb to higher oxidation states.
Keywords: heme oxygenase; hemoglobin; hemolysis; lung epithelium; mitochondria.
Figures


References
-
- Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal. 2010;13:1087–1123. - PubMed
-
- Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, Schaffner A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373–380. - PubMed
-
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. - PubMed
-
- Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

