Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1
- PMID: 26974308
- PMCID: PMC4823176
- DOI: 10.1038/nm.4059
Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1
Erratum in
-
Corrigendum: Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1.Nat Med. 2016 Jun 7;22(6):692. doi: 10.1038/nm0616-692a. Nat Med. 2016. PMID: 27270778 No abstract available.
Abstract
Tissues with high metabolic rates often use lipids, as well as glucose, for energy, conferring a survival advantage during feast and famine. Current dogma suggests that high-energy-consuming photoreceptors depend on glucose. Here we show that the retina also uses fatty acid β-oxidation for energy. Moreover, we identify a lipid sensor, free fatty acid receptor 1 (Ffar1), that curbs glucose uptake when fatty acids are available. Very-low-density lipoprotein receptor (Vldlr), which is present in photoreceptors and is expressed in other tissues with a high metabolic rate, facilitates the uptake of triglyceride-derived fatty acid. In the retinas of Vldlr(-/-) mice with low fatty acid uptake but high circulating lipid levels, we found that Ffar1 suppresses expression of the glucose transporter Glut1. Impaired glucose entry into photoreceptors results in a dual (lipid and glucose) fuel shortage and a reduction in the levels of the Krebs cycle intermediate α-ketoglutarate (α-KG). Low α-KG levels promotes stabilization of hypoxia-induced factor 1a (Hif1a) and secretion of vascular endothelial growth factor A (Vegfa) by starved Vldlr(-/-) photoreceptors, leading to neovascularization. The aberrant vessels in the Vldlr(-/-) retinas, which invade normally avascular photoreceptors, are reminiscent of the vascular defects in retinal angiomatous proliferation, a subset of neovascular age-related macular degeneration (AMD), which is associated with high vitreous VEGFA levels in humans. Dysregulated lipid and glucose photoreceptor energy metabolism may therefore be a driving force in macular telangiectasia, neovascular AMD and other retinal diseases.
Figures

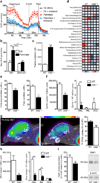
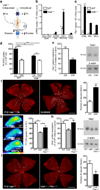
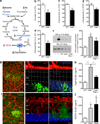
Comment in
-
Burning fat fuels photoreceptors.Nat Med. 2016 Apr;22(4):342-3. doi: 10.1038/nm.4080. Nat Med. 2016. PMID: 27050587 No abstract available.
References
-
- Cahill GF. Starvation in man. N Engl J Med. 1970;282:668–675. - PubMed
-
- Cohen LH, Noell WK. Glucose catabolism of rabbit retina before and after development of visual function. J Neurochem. 1960;5:253–276. - PubMed
-
- Goudriaan JR, et al. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res. 2004;45:1475–1481. - PubMed
METHODS-ONLY REFERENCES
-
- Spahis S, et al. Plasma fatty acid composition in French-Canadian children with non-alcoholic fatty liver disease: Effect of n-3 PUFA supplementation. Prostaglandins Leukot Essent Fatty Acids. 2015;99:25–34. - PubMed
-
- Calvano SE, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY017017/EY/NEI NIH HHS/United States
- R01 EY017017/EY/NEI NIH HHS/United States
- EY024963/EY/NEI NIH HHS/United States
- R01 EY022275/EY/NEI NIH HHS/United States
- P01 HD18655/HD/NICHD NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- R01 EY011254/EY/NEI NIH HHS/United States
- EY024864/EY/NEI NIH HHS/United States
- R01 EY024963/EY/NEI NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- EY11254/EY/NEI NIH HHS/United States
- EY022275/EY/NEI NIH HHS/United States
- R24 EY024864/EY/NEI NIH HHS/United States
- 143077/CAPMC/ CIHR/Canada
- R01 EY008670/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

