PTEN opposes negative selection and enables oncogenic transformation of pre-B cells
- PMID: 26974310
- PMCID: PMC5178869
- DOI: 10.1038/nm.4062
PTEN opposes negative selection and enables oncogenic transformation of pre-B cells
Abstract
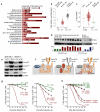
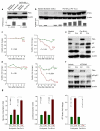
Phosphatase and tensin homolog (PTEN) is a negative regulator of the phosphatidylinositol 3-kinase (PI3K) and protein kinase B (AKT) signaling pathway and a potent tumor suppressor in many types of cancer. To test a tumor suppressive role for PTEN in pre-B acute lymphoblastic leukemia (ALL), we induced Cre-mediated deletion of Pten in mouse models of pre-B ALL. In contrast to its role as a tumor suppressor in other cancers, loss of one or both alleles of Pten caused rapid cell death of pre-B ALL cells and was sufficient to clear transplant recipient mice of leukemia. Small-molecule inhibition of PTEN in human pre-B ALL cells resulted in hyperactivation of AKT, activation of the p53 tumor suppressor cell cycle checkpoint and cell death. Loss of PTEN function in pre-B ALL cells was functionally equivalent to acute activation of autoreactive pre-B cell receptor signaling, which engaged a deletional checkpoint for the removal of autoreactive B cells. We propose that targeted inhibition of PTEN and hyperactivation of AKT triggers a checkpoint for the elimination of autoreactive B cells and represents a new strategy to overcome drug resistance in human ALL.
Figures




Comment in
-
PTEN enables the development of pre-B acute lymphoblastic leukemia.Nat Med. 2016 Apr;22(4):339-40. doi: 10.1038/nm.4083. Nat Med. 2016. PMID: 27050585 No abstract available.
Similar articles
-
Adult B-cell acute lymphoblastic leukemia cells display decreased PTEN activity and constitutive hyperactivation of PI3K/Akt pathway despite high PTEN protein levels.Haematologica. 2014 Jun;99(6):1062-8. doi: 10.3324/haematol.2013.096438. Epub 2014 Feb 21. Haematologica. 2014. PMID: 24561792 Free PMC article.
-
The relevance of PTEN-AKT in relation to NOTCH1-directed treatment strategies in T-cell acute lymphoblastic leukemia.Haematologica. 2016 Sep;101(9):1010-7. doi: 10.3324/haematol.2016.146381. Haematologica. 2016. PMID: 27582570 Free PMC article. Review.
-
MicroRNA-146b promotes PI3K/AKT pathway hyperactivation and thyroid cancer progression by targeting PTEN.Oncogene. 2018 Jun;37(25):3369-3383. doi: 10.1038/s41388-017-0088-9. Epub 2018 Jan 22. Oncogene. 2018. PMID: 29353884
-
PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability.J Clin Invest. 2008 Nov;118(11):3762-74. doi: 10.1172/JCI34616. Epub 2008 Oct 1. J Clin Invest. 2008. PMID: 18830414 Free PMC article.
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
Cited by
-
Philadelphia Chromosome-Positive Leukemia in the Lymphoid Lineage-Similarities and Differences with the Myeloid Lineage and Specific Vulnerabilities.Int J Mol Sci. 2020 Aug 12;21(16):5776. doi: 10.3390/ijms21165776. Int J Mol Sci. 2020. PMID: 32806528 Free PMC article. Review.
-
B-Cell-Specific Diversion of Glucose Carbon Utilization Reveals a Unique Vulnerability in B Cell Malignancies.Cell. 2018 Apr 5;173(2):470-484.e18. doi: 10.1016/j.cell.2018.02.048. Epub 2018 Mar 15. Cell. 2018. PMID: 29551267 Free PMC article.
-
B Cell Metabolism and Autophagy in Autoimmunity.Front Immunol. 2021 Jun 7;12:681105. doi: 10.3389/fimmu.2021.681105. eCollection 2021. Front Immunol. 2021. PMID: 34163480 Free PMC article. Review.
-
Aberrant B Cell Signaling in Autoimmune Diseases.Cells. 2022 Oct 27;11(21):3391. doi: 10.3390/cells11213391. Cells. 2022. PMID: 36359789 Free PMC article. Review.
-
PTEN-Regulated AID Transcription in Germinal Center B Cells Is Essential for the Class-Switch Recombination and IgG Antibody Responses.Front Immunol. 2018 Feb 28;9:371. doi: 10.3389/fimmu.2018.00371. eCollection 2018. Front Immunol. 2018. PMID: 29541074 Free PMC article.
References
-
- Osmond DG. Proliferation kinetics and the lifespan of B cells in central and peripheral lymphoid organs. Curr Opin Immunol. 1991;3:179–185. - PubMed
-
- Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science.; 2003;301:1374–1377. - PubMed
-
- Keenan RA, et al. Censoring of autoreactive B cell development by the pre-B cell receptor. Science.; 2008;321:696–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1TR000124/TR/NCATS NIH HHS/United States
- R01 CA213138/CA/NCI NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- R01 CA157644/CA/NCI NIH HHS/United States
- R01 CA172558/CA/NCI NIH HHS/United States
- R35 CA197628/CA/NCI NIH HHS/United States
- R01CA169458/CA/NCI NIH HHS/United States
- R01CA172558/CA/NCI NIH HHS/United States
- R01CA139032/CA/NCI NIH HHS/United States
- R01 CA137060/CA/NCI NIH HHS/United States
- R01CA137060/CA/NCI NIH HHS/United States
- R01 CA169458/CA/NCI NIH HHS/United States
- R01 CA139032/CA/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01CA157644/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

