Cause and management of muscle wasting in chronic liver disease
- PMID: 26974417
- PMCID: PMC5653274
- DOI: 10.1097/MOG.0000000000000261
Cause and management of muscle wasting in chronic liver disease
Abstract
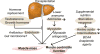
Purpose of review: Sarcopenia or loss of skeletal muscle mass is the major component of malnutrition and occurs in the majority of patients with liver disease. Lower muscle contractile function also contributes to the adverse consequences of sarcopenia. There are no effective therapies to prevent or reverse sarcopenia in liver disease. This review will discuss the advances in diagnosis, pathogenesis, and treatment options for sarcopenia in liver disease.
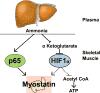
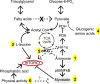
Recent findings: Sarcopenia increases mortality and risk of development of other complications of cirrhosis, and worsens postliver transplant outcomes while quality of life is decreased. Unlike other complications of cirrhosis that reverse after liver transplantation, sarcopenia may not improve and actually worsens. Impaired skeletal muscle protein synthesis and increased proteolysis via autophagy contribute to sarcopenia. Hyperammonemia is the best-studied mediator of the liver-muscle axis. Molecular studies show increased expression of myostatin whereas metabolic studies show impaired mitochondrial function and tricarboxylic acid cycle intermediates because of cataplerosis of α-ketoglutarate. Impaired skeletal muscle pyruvate and fatty acid oxidation during hyperammonemia suggest amino acids are diverted to acetyl CoA and potentially aggravate hyperammonemia. Nutritional supplementation is of limited or no benefit and suggests that cirrhosis is a state of anabolic resistance. Exercise may be beneficial but whether it overcomes anabolic resistance is not known.
Summary: The high clinical significance of sarcopenia is well established. Current approaches to nutritional supplementation have not been effective in reversing sarcopenia because of anabolic resistance. Myostatin antagonists, specific amino acid supplementation, mitochondrial protection, and combination endurance-resistance exercise are potential future therapeutic options.
Conflict of interest statement
Conflicts of interest: The author has no conflict of interest to declare.
Figures
References
-
- Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. - PubMed
-
- Bergerson JT, Lee JG, Furlan A, Sourianarayanane A, Fetzer DT, Tevar AD, Landsittel DP, DiMartini AF, Dunn MA. Liver transplantation arrests and reverses muscle wasting. Clin Transplant. 2015;29:216–221. - PubMed
-
- Dam G, Ott P, Aagaard NK, Vilstrup H. Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab Brain Dis. 2013;28:217–220. - PubMed
-
- Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int. 2011;31:1250–1258. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

