Sodium Selenite Acts as an Otoprotectant against Neomycin-Induced Hair Cell Damage in a Zebrafish Model
- PMID: 26974429
- PMCID: PMC4790947
- DOI: 10.1371/journal.pone.0151557
Sodium Selenite Acts as an Otoprotectant against Neomycin-Induced Hair Cell Damage in a Zebrafish Model
Abstract
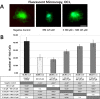
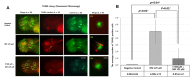
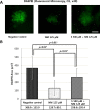
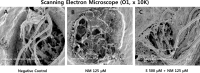
Sodium selenite is a trace element essential for many physiological functions in the body. It is involved in various biological processes; it acts as a cofactor for antioxidant enzymes that protect against free radicals and is reported to limit metal-mediated oxidative DNA damage. In the present study, we investigated the effect of sodium selenite on neomycin ototoxicity in wild-type and transgenic zebrafish (Brn3C: EGFP). Five or six days post-fertilization, zebrafish larvae were co-exposed to 125 μM neomycin and various concentrations (10 μM, 100 μM, 250 μM, and 500 μM) of sodium selenite for 1 h. Hair cells within neuromasts of the supraorbital (SO1 and SO2), otic (O1), and occipital (OC1) lateral lines were analyzed by fluorescence microscopy (n = 10 fish per treatment). Hair cell survival was estimated as the ratio of the hair cell numbers in each group compared to those of the control group that were not exposed to neomycin. Apoptosis and hair cell damage of neuromasts were evaluated using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) assay and 2-[4-(dimethylamino) styryl]-N-ethylpyridinium iodide (DASPEI) assay, respectively. Ultrastructural changes were evaluated using scanning electron microscopy and transmission electron microscopy. Neuromast hair cells were preserved in zebrafish exposed to 125 μM neomycin and 500 μM sodium selenite for 1 h. Sodium selenite protected against neomycin-induced hair cell loss of neuromasts, reduced apoptosis, and prevented zebrafish ultrastructural changes. We propose that sodium selenite protects against neomycin-induced hair cell damage by inhibiting apoptosis, decreasing the disarray of stereocilia, and preventing ultrastructural changes in the neuromast hair cells of the zebrafish.
Conflict of interest statement
Figures


Similar articles
-
Protective role of NecroX-5 against neomycin-induced hair cell damage in zebrafish.Arch Toxicol. 2014 Feb;88(2):435-41. doi: 10.1007/s00204-013-1124-3. Epub 2013 Sep 13. Arch Toxicol. 2014. PMID: 24030356
-
Protective effects of caffeic acid phenethyl ester (CAPE) against neomycin-induced hair cell damage in zebrafish.Int J Pediatr Otorhinolaryngol. 2014 Aug;78(8):1311-5. doi: 10.1016/j.ijporl.2014.05.018. Epub 2014 May 24. Int J Pediatr Otorhinolaryngol. 2014. PMID: 24880922
-
Protective effects of edaravone against cisplatin-induced hair cell damage in zebrafish.Int J Pediatr Otorhinolaryngol. 2013 Jun;77(6):1025-31. doi: 10.1016/j.ijporl.2013.04.003. Epub 2013 Apr 28. Int J Pediatr Otorhinolaryngol. 2013. PMID: 23628221
-
Zebrafish neuromast sensory system: Is it an emerging target to assess environmental pollution impacts?Environ Pollut. 2024 Mar 1;344:123400. doi: 10.1016/j.envpol.2024.123400. Epub 2024 Jan 23. Environ Pollut. 2024. PMID: 38272167 Review.
-
Chemical screening for hair cell loss and protection in the zebrafish lateral line.Zebrafish. 2010 Mar;7(1):3-11. doi: 10.1089/zeb.2009.0639. Zebrafish. 2010. PMID: 20192852 Free PMC article. Review.
Cited by
-
Protective Effects of Fasudil Against Cisplatin-Induced Ototoxicity in Zebrafish: An In Vivo Study.Int J Mol Sci. 2024 Dec 13;25(24):13363. doi: 10.3390/ijms252413363. Int J Mol Sci. 2024. PMID: 39769128 Free PMC article.
-
Investigation of structural, optical, photocatalytic, and antibacterial properties of ZnO doped GO nanoparticles for environment applications.Microsc Res Tech. 2025 Jan;88(1):73-91. doi: 10.1002/jemt.24672. Epub 2024 Aug 27. Microsc Res Tech. 2025. PMID: 39192686 Free PMC article.
-
Chemical screen in zebrafish lateral line identified compounds that ameliorate neomycin-induced ototoxicity by inhibiting ferroptosis pathway.Cell Biosci. 2024 Jun 5;14(1):71. doi: 10.1186/s13578-024-01258-w. Cell Biosci. 2024. PMID: 38840194 Free PMC article.
-
Using the Zebrafish Lateral Line to Understand the Roles of Mitochondria in Sensorineural Hearing Loss.Front Cell Dev Biol. 2021 Feb 5;8:628712. doi: 10.3389/fcell.2020.628712. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33614633 Free PMC article. Review.
-
Photosubstitution in a trisheteroleptic ruthenium complex inhibits conjunctival melanoma growth in a zebrafish orthotopic xenograft model.Chem Sci. 2022 May 16;13(23):6899-6919. doi: 10.1039/d2sc01646j. eCollection 2022 Jun 15. Chem Sci. 2022. PMID: 35774173 Free PMC article.
References
-
- Rybak LP (2005) Vestibular and auditory toxicity In: Cummings CW, Flint PW, Harker LA, Haughey BH, Richardson M.A., Robbins K.T., et al., editors. Otolaryngology & Head and Neck Surgery: Mosby; pp. 2933–2943
-
- Selimoglu E, Kalkandelen S, Erdogan F (2003) Comparative vestibulotoxicity of different aminoglycosides in the Guinea pigs. Yonsei Med J 44: 517–522. - PubMed
-
- Rostein CM, Lionel (2004) Clincal aminoglycoside ototoxicity In: Ronald PSR, John A. , editor Ototoxicity. London: Decker; pp. 81–92.
-
- Govaerts PJ, Claes J, van de Heyning PH, Jorens PG, Marquet J, De Broe ME (1990) Aminoglycoside-induced ototoxicity. Toxicol Lett 52: 227–251. - PubMed
-
- Roland PS (2003) Characteristics of systemic and topical agents implicated in toxicity of the middle and inner ear. Ear Nose Throat J 82 Suppl 1: 3–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

