How the kinetochore couples microtubule force and centromere stretch to move chromosomes
- PMID: 26974660
- PMCID: PMC4814359
- DOI: 10.1038/ncb3323
How the kinetochore couples microtubule force and centromere stretch to move chromosomes
Abstract
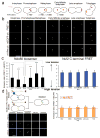
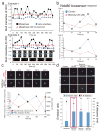
The Ndc80 complex (Ndc80, Nuf2, Spc24 and Spc25) is a highly conserved kinetochore protein essential for end-on anchorage to spindle microtubule plus ends and for force generation coupled to plus-end polymerization and depolymerization. Spc24/Spc25 at one end of the Ndc80 complex binds the kinetochore. The N-terminal tail and CH domains of Ndc80 bind microtubules, and an internal domain binds microtubule-associated proteins (MAPs) such as the Dam1 complex. To determine how the microtubule- and MAP-binding domains of Ndc80 contribute to force production at the kinetochore in budding yeast, we have inserted a FRET tension sensor into the Ndc80 protein about halfway between its microtubule-binding and internal loop domains. The data support a mechanical model of force generation at metaphase where the position of the kinetochore relative to the microtubule plus end reflects the relative strengths of microtubule depolymerization, centromere stretch and microtubule-binding interactions with the Ndc80 and Dam1 complexes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Wei RR, Al-Bassam J, Harrison SC. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nature structural & molecular biology. 2007;14:54–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

