Bacterial glycobiology: rhamnose-containing cell wall polysaccharides in Gram-positive bacteria
- PMID: 26975195
- PMCID: PMC4931226
- DOI: 10.1093/femsre/fuw006
Bacterial glycobiology: rhamnose-containing cell wall polysaccharides in Gram-positive bacteria
Abstract
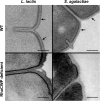
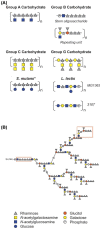
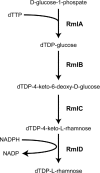
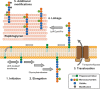
The composition of the Gram-positive cell wall is typically described as containing peptidoglycan, proteins and essential secondary cell wall structures called teichoic acids, which comprise approximately half of the cell wall mass. The cell walls of many species within the genera Streptococcus, Enterococcus and Lactococcus contain large amounts of the sugar rhamnose, which is incorporated in cell wall-anchored polysaccharides (CWP) that possibly function as homologues of well-studied wall teichoic acids (WTA). The presence and chemical structure of many rhamnose-containing cell wall polysaccharides (RhaCWP) has sometimes been known for decades. In contrast to WTA, insight into the biosynthesis and functional role of RhaCWP has been lacking. Recent studies in human streptococcal and enterococcal pathogens have highlighted critical roles for these complex polysaccharides in bacterial cell wall architecture and pathogenesis. In this review, we provide an overview of the RhaCWP with regards to their biosynthesis, genetics and biological function in species most relevant to human health. We also briefly discuss how increased knowledge in this field can provide interesting leads for new therapeutic compounds and improve biotechnological applications.
Keywords: Gram-positive bacteria; biosynthesis; cell wall polysaccharide; glycobiology; pathogenesis; rhamnose.
© FEMS 2016.
Figures


References
-
- Adibekian A, Stallforth P, Hecht M-L, et al. Comparative bioinformatics analysis of the mammalian and bacterial glycomes. Chem Sci. 2011;2:337–44.
-
- Alphey MS, Pirrie L, Torrie LS, et al. Allosteric competitive inhibitors of the glucose-1-phosphate thymidylyltransferase (RmlA) from Pseudomonas aeruginosa. ACS Chem Biol. 2013;8:387–96. - PubMed
-
- Ayoub EM, Dudding BA. Streptococcal Group A carbohydrate antibody in rheumatic and nonrheumatic bacterial endocarditis. J Lab Clin Med. 1970;76:322–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

