Metalloprotein Crystallography: More than a Structure
- PMID: 26975689
- PMCID: PMC4838947
- DOI: 10.1021/acs.accounts.5b00538
Metalloprotein Crystallography: More than a Structure
Abstract
Metal ions and metallocofactors play important roles in a broad range of biochemical reactions. Accordingly, it has been estimated that as much as 25-50% of the proteome uses transition metal ions to carry out a variety of essential functions. The metal ions incorporated within metalloproteins fulfill functional roles based on chemical properties, the diversity of which arises as transition metals can adopt different redox states and geometries, dictated by the identity of the metal and the protein environment. The coupling of a metal ion with an organic framework in metallocofactors, such as heme and cobalamin, further expands the chemical functionality of metals in biology. The three-dimensional visualization of metal ions and complex metallocofactors within a protein scaffold is often a starting point for enzymology, highlighting the importance of structural characterization of metalloproteins. Metalloprotein crystallography, however, presents a number of implicit challenges including correctly incorporating the relevant metal or metallocofactor, maintaining the proper environment for the protein to be purified and crystallized (including providing anaerobic, cold, or aphotic environments), and being mindful of the possibility of X-ray induced damage to the proteins or incorporated metal ions. Nevertheless, the incorporated metals or metallocofactors also present unique advantages in metalloprotein crystallography. The significant resonance that metals undergo with X-ray photons at wavelengths used for protein crystallography and the rich electronic properties of metals, which provide intense and spectroscopically unique signatures, allow a metalloprotein crystallographer to use anomalous dispersion to determine phases for structure solution and to use simultaneous or parallel spectroscopic techniques on single crystals. These properties, coupled with the improved brightness of beamlines, the ability to tune the wavelength of the X-ray beam, the availability of advanced detectors, and the incorporation of spectroscopic equipment at a number of synchrotron beamlines, have yielded exciting developments in metalloprotein structure determination. Here we will present results on the advantageous uses of metals in metalloprotein crystallography, including using metallocofactors to obtain phasing information, using K-edge X-ray absorption spectroscopy to identify metals coordinated in metalloprotein crystals, and using UV-vis spectroscopy on crystals to probe the enzymatic activity of the crystallized protein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

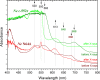
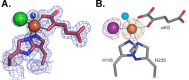
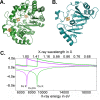
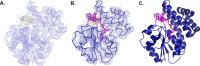
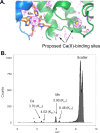
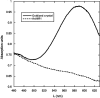
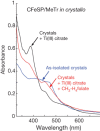
References
-
- Lippard S. J.; Berg J. M.. Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, 1994.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

