Age- and Hypertension-Associated Protein Aggregates in Mouse Heart Have Similar Proteomic Profiles
- PMID: 26975704
- PMCID: PMC4833546
- DOI: 10.1161/HYPERTENSIONAHA.115.06849
Age- and Hypertension-Associated Protein Aggregates in Mouse Heart Have Similar Proteomic Profiles
Abstract
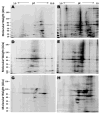
Neurodegenerative diseases are largely defined by protein aggregates in affected tissues. Aggregates contain some shared components as well as proteins thought to be specific for each disease. Aggregation has not previously been reported in the normal, aging heart or the hypertensive heart. Detergent-insoluble protein aggregates were isolated from mouse heart and characterized on 2-dimensional gels. Their levels increased markedly and significantly with aging and after sustained angiotensin II-induced hypertension. Of the aggregate components identified by high-resolution proteomics, half changed in abundance with age (392/787) or with sustained hypertension (459/824), whereas 30% (273/901) changed concordantly in both, each P<0.05. One fifth of these proteins were previously associated with age-progressive neurodegenerative or cardiovascular diseases, or both (eg, ApoE, ApoJ, ApoAIV, clusterin, complement C3, and others involved in stress-response and protein-homeostasis pathways). Because fibrosis is a characteristic of both aged and hypertensive hearts, we posited that aging of fibroblasts may contribute to the aggregates observed in cardiac tissue. Indeed, as cardiac myofibroblasts "senesced" (approached their replicative limit) in vitro, they accrued aggregates with many of the same constituent proteins observed in vivo during natural aging or sustained hypertension. In summary, we have shown for the first time that compact (detergent-insoluble) protein aggregates accumulate during natural aging, chronic hypertension, and in vitro myofibroblast senescence, sharing many common proteins. Thus, aggregates that arise from disparate causes (aging, hypertension, and replicative senescence) may have common underlying mechanisms of accrual.
Keywords: aging (cardiac); cardiovascular diseases; hypertension; neurodegenerative diseases; protein aggregates.
© 2016 American Heart Association, Inc.
Figures

References
-
- Soto C, Estrada LD. Protein misfolding and neurodegeneration. Arch Neurol. 2008;65:184–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30GM103450/GM/NIGMS NIH HHS/United States
- UL1 TR000039/TR/NCATS NIH HHS/United States
- UL1TR000039/TR/NCATS NIH HHS/United States
- P30-AG028718/AG/NIA NIH HHS/United States
- R01GM106024/GM/NIGMS NIH HHS/United States
- R01 GM106024/GM/NIGMS NIH HHS/United States
- I01 BX000282/BX/BLRD VA/United States
- I01 BX001655/BX/BLRD VA/United States
- P20 GM103429/GM/NIGMS NIH HHS/United States
- P30 AG028718/AG/NIA NIH HHS/United States
- R33CA173264/CA/NCI NIH HHS/United States
- P20GM103429/GM/NIGMS NIH HHS/United States
- P30 GM103450/GM/NIGMS NIH HHS/United States
- R33 CA173264/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

