Immunogenicity of a chimeric Plasmodium falciparum merozoite surface protein vaccine in Aotus monkeys
- PMID: 26975721
- PMCID: PMC4791798
- DOI: 10.1186/s12936-016-1226-5
Immunogenicity of a chimeric Plasmodium falciparum merozoite surface protein vaccine in Aotus monkeys
Abstract
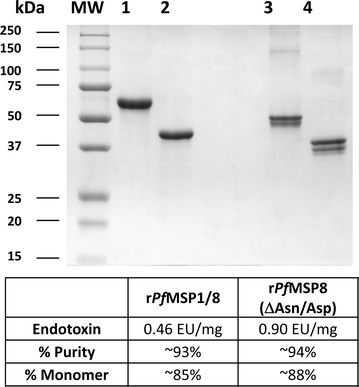
Background: The production of properly folded, recombinant sub-unit Plasmodium falciparum malaria vaccine candidates in sufficient quantities is often a challenge. Success in vaccine immunogenicity studies in small animal models does not always predict immunogenicity in non-human primates and/or human subjects. The aim of this study was to assess the immunogenicity of a chimeric blood-stage malaria vaccine in Aotus monkeys. This vaccine candidate includes the neutralizing B cell epitopes of P. falciparum merozoite surface protein 1 (rPfMSP119) genetically linked to a highly immunogenic, well-conserved P. falciparum merozoite surface protein 8 (rPfMSP8 (ΔAsn/Asp)) partner.
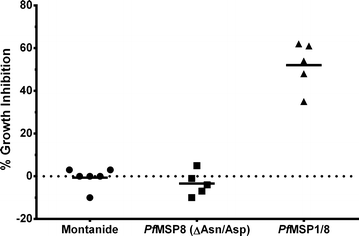
Methods: Aotus nancymaae monkeys were immunized with purified rPfMSP1/8 or rPfMSP8 (ΔAsn/Asp) formulated with Montanide ISA 720 as adjuvant, or with adjuvant alone. Antibody responses to MSP119 and MSP8 domains were measured by ELISA following primary, secondary and tertiary immunizations. The functionality of vaccine-induced antibodies was assessed in a standard P. falciparum blood-stage in vitro growth inhibition assay. Non-parametric tests with corrections for multiple comparisons when appropriate were used to determine the significance of differences in antigen-specific IgG titres and in parasite growth inhibition.
Results: The chimeric rPfMSP1/8 vaccine was shown to be well tolerated and highly immunogenic with boost-able antibody responses elicited to both PfMSP8 and PfMSP119 domains. Elicited antibodies were highly cross-reactive between FVO and 3D7 alleles of PfMSP119 and potently inhibited the in vitro growth of P. falciparum blood-stage parasites.
Conclusions: Similar to previous results with inbred and outbred mice and with rabbits, the PfMSP1/8 vaccine was shown to be highly effective in eliciting P. falciparum growth inhibitory antibodies upon immunization of non-human primates. The data support the further assessment of PfMSP1/8 as a component of a multivalent vaccine for use in human subjects. As important, the data indicate that rPfMSP8 (ΔAsn/Asp) can be used as a malaria specific carrier protein to: (1) drive production of antibody responses to neutralizing B cell epitopes of heterologous vaccine candidates and (2) facilitate production of properly folded, recombinant P. falciparum subunit vaccines in high yield.
Keywords: Aotus monkeys; Blood-stage malaria vaccine; Vaccine carrier protein.
Figures

References
-
- WHO. World Malaria Report 2014. Geneva: World Health Organization; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

