Optogenetic skeletal muscle-powered adaptive biological machines
- PMID: 26976577
- PMCID: PMC4822586
- DOI: 10.1073/pnas.1516139113
Optogenetic skeletal muscle-powered adaptive biological machines
Abstract
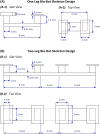
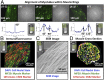
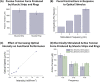
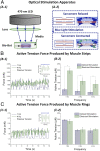
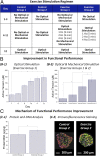
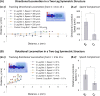
Complex biological systems sense, process, and respond to their surroundings in real time. The ability of such systems to adapt their behavioral response to suit a range of dynamic environmental signals motivates the use of biological materials for other engineering applications. As a step toward forward engineering biological machines (bio-bots) capable of nonnatural functional behaviors, we created a modular light-controlled skeletal muscle-powered bioactuator that can generate up to 300 µN (0.56 kPa) of active tension force in response to a noninvasive optical stimulus. When coupled to a 3D printed flexible bio-bot skeleton, these actuators drive directional locomotion (310 µm/s or 1.3 body lengths/min) and 2D rotational steering (2°/s) in a precisely targeted and controllable manner. The muscle actuators dynamically adapt to their surroundings by adjusting performance in response to "exercise" training stimuli. This demonstration sets the stage for developing multicellular bio-integrated machines and systems for a range of applications.
Keywords: bioactuator; soft robotics; stereolithography; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Griffith LG, Naughton G. Tissue engineering—Current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014. - PubMed
-
- Harrison RH, St-Pierre J-P, Stevens MM. Tissue engineering and regenerative medicine: A year in review. Tissue Eng Part B Rev. 2014;20(1):1–16. - PubMed
-
- Chan V, Asada HH, Bashir R. Utilization and control of bioactuators across multiple length scales. Lab Chip. 2014;14(4):653–670. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

