Four-dimensional maps of the human somatosensory system
- PMID: 26976579
- PMCID: PMC4822602
- DOI: 10.1073/pnas.1601889113
Four-dimensional maps of the human somatosensory system
Abstract
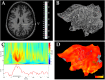
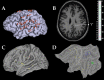
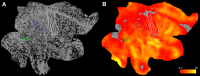
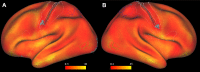
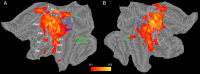
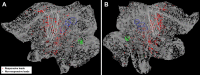
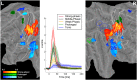
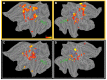
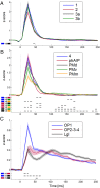
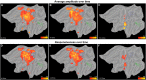
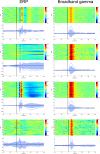
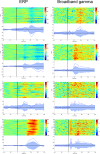
A fine-grained description of the spatiotemporal dynamics of human brain activity is a major goal of neuroscientific research. Limitations in spatial and temporal resolution of available noninvasive recording and imaging techniques have hindered so far the acquisition of precise, comprehensive four-dimensional maps of human neural activity. The present study combines anatomical and functional data from intracerebral recordings of nearly 100 patients, to generate highly resolved four-dimensional maps of human cortical processing of nonpainful somatosensory stimuli. These maps indicate that the human somatosensory system devoted to the hand encompasses a widespread network covering more than 10% of the cortical surface of both hemispheres. This network includes phasic components, centered on primary somatosensory cortex and neighboring motor, premotor, and inferior parietal regions, and tonic components, centered on opercular and insular areas, and involving human parietal rostroventral area and ventral medial-superior-temporal area. The technique described opens new avenues for investigating the neural basis of all levels of cortical processing in humans.
Keywords: cerebral cortex; intracranial recordings; median nerve; stereo-EEG; temporal dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Debener S, Ullsperger M, Siegel M, Engel AK. Single-trial EEG-fMRI reveals the dynamics of cognitive function. Trends Cogn Sci. 2006;10(12):558–563. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

