Evaluation of Commercially Available Chikungunya Virus Immunoglobulin M Detection Assays
- PMID: 26976887
- PMCID: PMC4944686
- DOI: 10.4269/ajtmh.16-0013
Evaluation of Commercially Available Chikungunya Virus Immunoglobulin M Detection Assays
Abstract
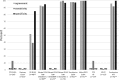
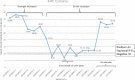
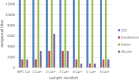
Commercial chikungunya virus (CHIKV)-specific IgM detection kits were evaluated at the Centers for Disease Control and Prevention (CDC), the Public Health Agency of Canada National Microbiology Laboratory, and the Caribbean Public Health Agency (CARPHA). The Euroimmun Anti-CHIKV IgM ELISA kit had ≥ 95% concordance with all three reference laboratory results. The limit of detection for low CHIK IgM+ samples, as measured by serial dilution of seven sera up to 1:12,800 ranged from 1:800 to 1:3,200. The Euroimmun IIFT kit evaluated at CDC and CARPHA performed well, but required more retesting of equivocal results. The InBios CHIKjj Detect MAC-ELISA had 100% and 98% concordance with CDC and CARPHA results, respectively, and had equal sensitivity to the CDC MAC-ELISA to 1:12,800 dilution in serially diluted samples. The Abcam Anti-CHIKV IgM ELISA had high performance at CARPHA, but at CDC, performance was inconsistent between lots. After replacement of the biotinylated IgM antibody controls with serum containing CHIKV-specific IgM and additional quality assurance/control measures, the Abcam kit was rereleased and reevaluated at CDC. The reformatted Abcam kit had 97% concordance with CDC results and limit of detection of 1:800 to 1:3,200. Two rapid tests and three other CHIKV MAC-ELISAs evaluated at CDC had low sensitivity, as the CDC CHIKV IgM in-house positive controls were below the level of detection. In conclusion, laboratories have options for CHIKV serological diagnosis using validated commercial kits.
© The American Society of Tropical Medicine and Hygiene.
Figures
Similar articles
-
Evaluation of Two Enzyme-Linked Immunosorbent Assay Kits for Chikungunya Virus IgM Using Samples from Deceased Organ and Tissue Donors.Clin Vaccine Immunol. 2016 Oct 4;23(10):825-830. doi: 10.1128/CVI.00330-16. Print 2016 Oct. Clin Vaccine Immunol. 2016. PMID: 27535838 Free PMC article.
-
Laboratory Diagnosis of Chikungunya Virus Infections and Commercial Sources for Diagnostic Assays.J Infect Dis. 2016 Dec 15;214(suppl 5):S471-S474. doi: 10.1093/infdis/jiw274. J Infect Dis. 2016. PMID: 27920176 Free PMC article.
-
Combining anti-IgM and IgG immunoassays for comprehensive chikungunya virus diagnostic testing.Zoonoses Public Health. 2019 Dec;66(8):909-917. doi: 10.1111/zph.12641. Epub 2019 Aug 26. Zoonoses Public Health. 2019. PMID: 31449360
-
Diagnostic accuracy of serological tests for the diagnosis of Chikungunya virus infection: A systematic review and meta-analysis.PLoS Negl Trop Dis. 2022 Feb 4;16(2):e0010152. doi: 10.1371/journal.pntd.0010152. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35120141 Free PMC article.
-
Luciferase Immunosorbent Assay Based on Multiple E Antigens for the Detection of Chikungunya Virus-Specific IgG Antibodies.Microbiol Spectr. 2022 Apr 27;10(2):e0149621. doi: 10.1128/spectrum.01496-21. Epub 2022 Mar 21. Microbiol Spectr. 2022. PMID: 35311573 Free PMC article. Review.
Cited by
-
Lack of Evidence of Chikungunya Virus Infection among Blood Donors during the Chikungunya Outbreak in Lazio Region, Italy, 2017.Viruses. 2022 Mar 16;14(3):619. doi: 10.3390/v14030619. Viruses. 2022. PMID: 35337026 Free PMC article.
-
Cellular and Molecular Immune Response to Chikungunya Virus Infection.Front Cell Infect Microbiol. 2018 Oct 10;8:345. doi: 10.3389/fcimb.2018.00345. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30364124 Free PMC article. Review.
-
Chikungunya E2 Protein Produced in E. coli and HEK293-T Cells-Comparison of Their Performances in ELISA.Viruses. 2020 Aug 26;12(9):939. doi: 10.3390/v12090939. Viruses. 2020. PMID: 32858804 Free PMC article.
-
Neurological Disease Associated with Chikungunya in Indonesia.Am J Trop Med Hyg. 2022 Jun 13;107(2):291-295. doi: 10.4269/ajtmh.22-0050. Print 2022 Aug 17. Am J Trop Med Hyg. 2022. PMID: 35895435 Free PMC article.
-
Surveillance of Vector-Borne Infections (Chikungunya, Dengue, and Malaria) in Bo, Sierra Leone, 2012-2013.Am J Trop Med Hyg. 2017 Oct;97(4):1151-1154. doi: 10.4269/ajtmh.16-0798. Am J Trop Med Hyg. 2017. PMID: 29031286 Free PMC article.
References
-
- Powers AM. Chikungunya. Clin Lab Med. 2010;30:209–219. - PubMed
-
- Powers AM, Roehrig JT. Alphaviruses. Methods Mol Biol. 2011;665:17–38. - PubMed
-
- Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

