Human DC-SIGN binds specific human milk glycans
- PMID: 26976925
- PMCID: PMC4875834
- DOI: 10.1042/BCJ20160046
Human DC-SIGN binds specific human milk glycans
Abstract
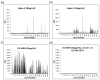
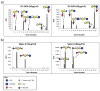
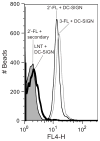
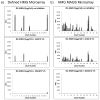
Human milk glycans (HMGs) are prebiotics, pathogen receptor decoys and regulators of host physiology and immune responses. Mechanistically, human lectins (glycan-binding proteins, hGBP) expressed by dendritic cells (DCs) are of major interest, as these cells directly contact HMGs. To explore such interactions, we screened many C-type lectins and sialic acid-binding immunoglobulin-like lectins (Siglecs) expressed by DCs for glycan binding on microarrays presenting over 200 HMGs. Unexpectedly, DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) showed robust binding to many HMGs, whereas other C-type lectins failed to bind, and Siglec-5 and Siglec-9 showed weak binding to a few glycans. By contrast, most hGBP bound to multiple glycans on other microarrays lacking HMGs. An α-linked fucose residue was characteristic of HMGs bound by DC-SIGN. Binding of DC-SIGN to the simple HMGs 2'-fucosyl-lactose (2'-FL) and 3-fucosyl-lactose (3-FL) was confirmed by flow cytometry to beads conjugated with 2'-FL or 3-FL, as well as the ability of the free glycans to inhibit DC-SIGN binding. 2'-FL had an IC50 of ∼1 mM for DC-SIGN, which is within the physiological concentration of 2'-FL in human milk. These results demonstrate that DC-SIGN among the many hGBP expressed by DCs binds to α-fucosylated HMGs, and suggest that such interactions may be important in influencing immune responses in the developing infant.
Keywords: dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN); glycan microarrays; glycan recognition; glycan-binding proteins; human milk glycans; lectins.
© 2016 The Author(s). published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
R.D.C. and D.F.S. are consultants for Abbott Nutrition. G.D.M. and R.H.B. are employees of Abbott. The other authors declare that they have no conflict of interest in the work reported.
Figures


References
-
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. - PubMed
-
- Newburg DS. Glycobiology of human milk. Biochemistry (Mosc) 2013;78:771–785. - PubMed
-
- Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

