Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes
- PMID: 26977395
- PMCID: PMC4770267
- DOI: 10.1016/j.molmet.2016.01.002
Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes
Abstract
Objective: Although glucagon-secreting α-cells and insulin-secreting β-cells have opposing functions in regulating plasma glucose levels, the two cell types share a common developmental origin and exhibit overlapping transcriptomes and epigenomes. Notably, destruction of β-cells can stimulate repopulation via transdifferentiation of α-cells, at least in mice, suggesting plasticity between these cell fates. Furthermore, dysfunction of both α- and β-cells contributes to the pathophysiology of type 1 and type 2 diabetes, and β-cell de-differentiation has been proposed to contribute to type 2 diabetes. Our objective was to delineate the molecular properties that maintain islet cell type specification yet allow for cellular plasticity. We hypothesized that correlating cell type-specific transcriptomes with an atlas of open chromatin will identify novel genes and transcriptional regulatory elements such as enhancers involved in α- and β-cell specification and plasticity.
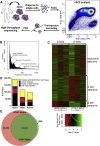
Methods: We sorted human α- and β-cells and performed the "Assay for Transposase-Accessible Chromatin with high throughput sequencing" (ATAC-seq) and mRNA-seq, followed by integrative analysis to identify cell type-selective gene regulatory regions.
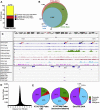
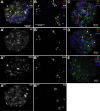
Results: We identified numerous transcripts with either α-cell- or β-cell-selective expression and discovered the cell type-selective open chromatin regions that correlate with these gene activation patterns. We confirmed cell type-selective expression on the protein level for two of the top hits from our screen. The "group specific protein" (GC; or vitamin D binding protein) was restricted to α-cells, while CHODL (chondrolectin) immunoreactivity was only present in β-cells. Furthermore, α-cell- and β-cell-selective ATAC-seq peaks were identified to overlap with known binding sites for islet transcription factors, as well as with single nucleotide polymorphisms (SNPs) previously identified as risk loci for type 2 diabetes.
Conclusions: We have determined the genetic landscape of human α- and β-cells based on chromatin accessibility and transcript levels, which allowed for detection of novel α- and β-cell signature genes not previously known to be expressed in islets. Using fine-mapping of open chromatin, we have identified thousands of potential cis-regulatory elements that operate in an endocrine cell type-specific fashion.
Keywords: ARX, aristaless related homeobox; ATAC-seq, Assay for Transposase-Accessible Chromatin with high throughput sequencing; Alpha cell; Beta cell; CHODL, chondrolectin; ChIP-seq, Chromatin Immunoprecipitation followed by high throughput sequencing; DAPI, 4′,6-diamidino-2-phenylindole; DPP4, dipeptidyl-peptidase 4; Diabetes; Epigenetics; FACS, fluorescence-activated cell sorting; FAIRE-seq, Formaldehyde-Assisted Isolation of Regulatory Elements followed by high throughput sequencing; GC, group-specific protein; GCG, glucagon; GHRL, ghrelin; IGF2, insulin like growth factor 2; INS, insulin; IRX2, iroquois homeobox 2; Islet; MAFA, v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog A; NEUROD1, neuronal differentiation 1; Open chromatin; PP, pancreatic polypeptide; SNP, single nucleotide polymorphism; SST, somatostatin.
Figures




References
-
- Brissova M., Fowler M.J., Nicholson W.E., Chu A., Hirshberg B., Harlan D.M. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. Journal of Histochemistry & Cytochemistry. 2005;53(9):1087–1097. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

