Immunoadsorption therapy in autoimmune encephalitides
- PMID: 26977423
- PMCID: PMC4772911
- DOI: 10.1212/NXI.0000000000000207
Immunoadsorption therapy in autoimmune encephalitides
Abstract
Objective: It was hypothesized that in encephalitides with autoantibodies directed to CNS surface antigens an antibody-removing intervention might speed up recovery.
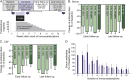
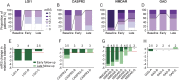
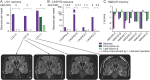
Methods: The outcome of autoimmune encephalitis in 19 patients with antibodies against surface antigens (leucine-rich, glioma inactivated 1 [LGI1], n = 3; contactin-associated protein-2 [CASPR2], n = 4; NMDA receptor [NMDAR], n = 7) and intracellular antigens (glutamic acid decarboxylase [GAD], n = 5) after immunoadsorption in addition to corticosteroid therapy was evaluated retrospectively. Modified Rankin scale (mRS) scores and data on seizures, memory, and antibody titers directly after immunoadsorption (early follow-up) and after a median of 4 months (late follow-up) were compiled.
Results: Immediately after immunoadsorption, 9 of 14 patients with antibodies against LGI1, CASPR2, or NMDAR (64%), but none with GAD antibodies, had improved by at least one mRS point. Five of the 7 patients with LGI1 or CASRP2 antibodies had become seizure-free, and 2 patients with NMDAR antibodies had a memory improvement of more than 1 SD of a normal control population. At late follow-up, 12 of 14 patients with surface antibodies had improved (86%), and none of the patients with GAD antibodies.
Conclusions: It is suggested that addition of immunoadsorption to immunosuppression therapy in patients with surface antibodies may accelerate recovery. This supports the pathogenic role of surface antibodies.
Classification of evidence: This study provides Class IV evidence that immunoadsorption combined with immunosuppression therapy is effective in patients with autoimmune encephalitis with surface antibodies.
Figures
References
-
- Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol 2011;10:759–772. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
