Time-Resolved Visualisation of Nearly-Native Influenza A Virus Progeny Ribonucleoproteins and Their Individual Components in Live Infected Cells
- PMID: 26978069
- PMCID: PMC4792379
- DOI: 10.1371/journal.pone.0149986
Time-Resolved Visualisation of Nearly-Native Influenza A Virus Progeny Ribonucleoproteins and Their Individual Components in Live Infected Cells
Abstract
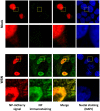
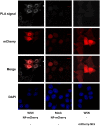
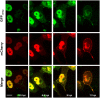
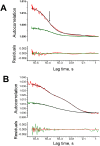
Influenza viruses are a global health concern because of the permanent threat of novel emerging strains potentially capable of causing pandemics. Viral ribonucleoproteins (vRNPs) containing genomic RNA segments, nucleoprotein oligomers, and the viral polymerase, play a central role in the viral replication cycle. Our knowledge about critical events such as vRNP assembly and interactions with other viral and cellular proteins is poor and could be substantially improved by time lapse imaging of the infected cells. However, such studies are limited by the difficulty to achieve live-cell compatible labeling of active vRNPs. Previously we designed the first unimpaired recombinant influenza WSN-PB2-GFP11 virus allowing fluorescent labeling of the PB2 subunit of the viral polymerase (Avilov et al., J.Virol. 2012). Here, we simultaneously labeled the viral PB2 protein using the above-mentioned strategy, and virus-encoded progeny RNPs through spontaneous incorporation of transiently expressed NP-mCherry fusion proteins during RNP assembly in live infected cells. This dual labeling enabled us to visualize progeny vRNPs throughout the infection cycle and to characterize independently the mobility, oligomerization status and interactions of vRNP components in the nuclei of live infected cells.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

