Safety and Efficacy of Memantine in Children with Autism: Randomized, Placebo-Controlled Study and Open-Label Extension
- PMID: 26978327
- PMCID: PMC5510039
- DOI: 10.1089/cap.2015.0146
Safety and Efficacy of Memantine in Children with Autism: Randomized, Placebo-Controlled Study and Open-Label Extension
Abstract
Objective: Abnormal glutamatergic neurotransmission is implicated in the pathophysiology of autism spectrum disorder (ASD). In this study, the safety, tolerability, and efficacy of the glutamatergic N-methyl-d-aspartate (NMDA) receptor antagonist memantine (once-daily extended-release [ER]) were investigated in children with autism in a randomized, placebo-controlled, 12 week trial and a 48 week open-label extension.
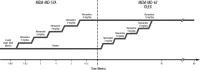
Methods: A total of 121 children 6-12 years of age with Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR)-defined autistic disorder were randomized (1:1) to placebo or memantine ER for 12 weeks; 104 children entered the subsequent extension trial. Maximum memantine doses were determined by body weight and ranged from 3 to 15 mg/day.
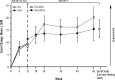
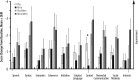
Results: There was one serious adverse event (SAE) (affective disorder, with memantine) in the 12 week study and one SAE (lobar pneumonia) in the 48 week extension; both were deemed unrelated to treatment. Other AEs were considered mild or moderate and most were deemed not related to treatment. No clinically significant changes occurred in clinical laboratory values, vital signs, or electrocardiogram (ECG). There was no significant between-group difference on the primary efficacy outcome of caregiver/parent ratings on the Social Responsiveness Scale (SRS), although an improvement over baseline at Week 12 was observed in both groups. A trend for improvement at the end of the 48 week extension was observed. No improvements in the active group were observed on any of the secondary end-points, with one communication measure showing significant worsening with memantine compared with placebo (p = 0.02) after 12 weeks.
Conclusions: This trial did not demonstrate clinical efficacy of memantine ER in autism; however, the tolerability and safety data were reassuring. Our results could inform future trial design in this population and may facilitate the investigation of memantine ER for other clinical applications.
Keywords: Children's Communication Checklist-2; Social Responsiveness Scale; autistic disorder; core symptoms of autism; memantine extended release; tolerability, safety, and adverse events.
Conflict of interest statement
In the past 36 months, Dr. Aman received research contracts from, consulted with, served on advisory boards of, or performed investigator training for Biomarin Pharmaceuticals, Bristol-Myers Squibb, CogState, Inc., CogState Clinical Trials, Ltd., Confluence Pharmaceutica, Coronado Biosciences, Forest Research Institute, Hoffman-La Roche, Janssen Pharmaceuticals, Johnson & Johnson, Lumos Pharma, MedAvante, Inc., Novartis, Pfizer, ProPhase LLC, and Supernus Pharmaceuticals. Dr. Findling receives or has received research support from, or acted as a consultant and/or served on a speaker's bureau for Alcobra, American Academy of Child & Adolescent Psychiatry, American Physician Institute, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, CogCubed, Cognition Group, Coronado Biosciences, Dana Foundation, Elsevier, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson and Johnson, Jubilant Clinsys, KemPharm, Lilly, Lundbeck, Merck, NIH, Neurim, Novartis, Noven, Otsuka, Oxford University Press, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD. Dr. Hardan previously received research funding from Forest; he has no current conflicts of interest to disclose. Dr. Hendren received research grants from Autism Speaks, BioMarin Pharmaceuticals, Curemark, Forest, Roche, Shire, Sunovion, and the Vitamin D Council; is on Advisory Boards for BioMarin Pharmaceuticals, Forest, Janssen, and Neuren; and is a reviewer for Autism Speaks, Brain Canada, and Simons Foundation. Dr. Melmed received research support from Alcobra, Bristol‐Myers Squibb Company, Forest Laboratories Inc, Neurim, Novartis Pharmaceuticals, Roche Pharmaceuticals, and Seaside Pharmaceuticals during the past year. Ms. Kehinde-Nelson, and Drs. Hsu, Trugman, Palmer, Graham, Gage, Perhach, and Katz were employees of the study sponsor at the time of the study and are present or former stockholders of Forest (now Allergan). Dr. Trugman is currently an employee of the study sponsor.
Figures
References
-
- Aman MG, Singh NN, Stewart AW, Field CJ: The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Defic 89:485–491, 1985 - PubMed
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000
-
- Bauman ML, Kemper TL: Neuroanatomic observations of the brain in autism: A review and future directions. Int J Dev Neurosci 23:183–187, 2005 - PubMed
-
- Bishop DVM: Children's Communication Checklist-2 (CCC-2) US Edition (Manual). San Antonio: Harcourt Assessments, Inc.; 2006
-
- Blue ME, Naidu S, Johnston MV: Development of amino acid receptors in frontal cortex from girls with Rett syndrome. Ann Neurol 45:541–545, 1999 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
