Immune Complexes Indirectly Suppress the Generation of Th17 Responses In Vivo
- PMID: 26978520
- PMCID: PMC4792482
- DOI: 10.1371/journal.pone.0151252
Immune Complexes Indirectly Suppress the Generation of Th17 Responses In Vivo
Abstract
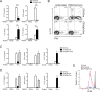
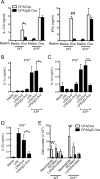
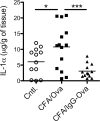
The precise context in which the innate immune system is activated plays a pivotal role in the subsequent instruction of CD4+ T helper (Th) cell responses. Th1 responses are downregulated when antigen is encountered in the presence of antigen-IgG immune complexes. To assess if Th17 responses to antigen are subject to similar influences in the presence of immune complexes we utilized an inflammatory airway disease model in which immunization of mice with Complete Freund's Adjuvant (CFA) and ovalbumin (Ova) induces a powerful Ova-specific Th1 and Th17 response. Here we show that modification of that immunization with CFA to include IgG-Ova immune complexes results in the suppression of CFA-induced Th17 responses and a concurrent enhancement of Ova-specific Th2 responses. Furthermore, we show the mechanism by which these immune complexes suppress Th17 responses is through the enhancement of IL-10 production. In addition, the generation of Th17 responses following immunization with CFA and Ova were dependent on IL-1α but independent of NLRP3 inflammasome activation. Together these data represent a novel mechanism by which the generation of Th17 responses is regulated.
Conflict of interest statement
Figures




Similar articles
-
Enhancement of ovalbumin-specific Th1, Th2, and Th17 immune responses by amorphous silica nanoparticles.Int J Immunopathol Pharmacol. 2016 Sep;29(3):408-20. doi: 10.1177/0394632016656192. Epub 2016 Jun 24. Int J Immunopathol Pharmacol. 2016. PMID: 27343242 Free PMC article.
-
Streptococcus pneumoniae fructose-1,6-bisphosphate aldolase, a protein vaccine candidate, elicits Th1/Th2/Th17-type cytokine responses in mice.Int J Mol Med. 2016 Apr;37(4):1127-38. doi: 10.3892/ijmm.2016.2512. Epub 2016 Mar 1. Int J Mol Med. 2016. PMID: 26935978
-
Immune effector cells induced by complete Freund's adjuvant exert an inhibitory effect on antigen-specific type 2 T helper responses.Clin Exp Allergy. 1997 Mar;27(3):315-24. Clin Exp Allergy. 1997. PMID: 9088658
-
Inflammasome-IL-1-Th17 response in allergic lung inflammation.J Mol Cell Biol. 2012 Feb;4(1):3-10. doi: 10.1093/jmcb/mjr042. Epub 2011 Dec 6. J Mol Cell Biol. 2012. PMID: 22147847 Review.
-
Inflammasome Contribution to the Activation of Th1, Th2, and Th17 Immune Responses.Front Microbiol. 2022 Mar 17;13:851835. doi: 10.3389/fmicb.2022.851835. eCollection 2022. Front Microbiol. 2022. PMID: 35369454 Free PMC article. Review.
Cited by
-
The guanine nucleotide exchange factor Rin-like controls Tfh cell differentiation via CD28 signaling.J Exp Med. 2023 Nov 6;220(11):e20221466. doi: 10.1084/jem.20221466. Epub 2023 Sep 13. J Exp Med. 2023. PMID: 37703004 Free PMC article.
-
BJ-1108, a 6-Amino-2,4,5-trimethylpyridin-3-ol analogue, regulates differentiation of Th1 and Th17 cells to ameliorate experimental autoimmune encephalomyelitis.Biol Res. 2017 Feb 28;50(1):8. doi: 10.1186/s40659-017-0113-z. Biol Res. 2017. PMID: 28241881 Free PMC article.
-
Amelioration of Experimental autoimmune encephalomyelitis and DSS induced colitis by NTG-A-009 through the inhibition of Th1 and Th17 cells differentiation.Sci Rep. 2018 May 17;8(1):7799. doi: 10.1038/s41598-018-26088-y. Sci Rep. 2018. PMID: 29773813 Free PMC article.
-
Understanding Host Immunity and the Gut Microbiota Inspires the New Development of Vaccines and Adjuvants.Pharmaceutics. 2021 Jan 26;13(2):163. doi: 10.3390/pharmaceutics13020163. Pharmaceutics. 2021. PMID: 33530627 Free PMC article. Review.
-
Sphingosine 1-Phosphate Receptor 1 Signaling Maintains Endothelial Cell Barrier Function and Protects Against Immune Complex-Induced Vascular Injury.Arthritis Rheumatol. 2018 Nov;70(11):1879-1889. doi: 10.1002/art.40558. Arthritis Rheumatol. 2018. PMID: 29781582 Free PMC article.
References
-
- Anderson CF, Lucas M, Gutierrez-Kobeh L, Field AE, Mosser DM. T cell biasing by activated dendritic cells. J Immunol. 2004;173(2):955–61. Epub 2004/07/09. . - PubMed
-
- Bandukwala HS, Clay BS, Tong J, Mody PD, Cannon JL, Shilling RA, et al. Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med. 2007;204(8):1875–89. Epub 2007/08/01. doi: jem.20061134 [pii] 10.1084/jem.20061134 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

