Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent Versus Durable-Polymer Everolimus-Eluting Stent for Percutaneous Coronary Revascularization: 2-Year Results of the BIOSCIENCE Trial
- PMID: 26979080
- PMCID: PMC4943287
- DOI: 10.1161/JAHA.116.003255
Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent Versus Durable-Polymer Everolimus-Eluting Stent for Percutaneous Coronary Revascularization: 2-Year Results of the BIOSCIENCE Trial
Abstract
Background: No data are available on the long-term performance of ultrathin strut biodegradable polymer sirolimus-eluting stents (BP-SES). We reported 2-year clinical outcomes of the BIOSCIENCE (Ultrathin Strut Biodegradable Polymer Sirolimus-Eluting Stent Versus Durable Polymer Everolimus-Eluting Stent for Percutaneous Coronary Revascularisation) trial, which compared BP-SES with durable-polymer everolimus-eluting stents (DP-EES) in patients undergoing percutaneous coronary intervention.
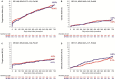
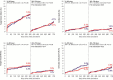
Methods and results: A total of 2119 patients with minimal exclusion criteria were assigned to treatment with BP-SES (n=1063) or DP-EES (n=1056). Follow-up at 2 years was available for 2048 patients (97%). The primary end point was target-lesion failure, a composite of cardiac death, target-vessel myocardial infarction, or clinically indicated target-lesion revascularization. At 2 years, target-lesion failure occurred in 107 patients (10.5%) in the BP-SES arm and 107 patients (10.4%) in the DP-EES arm (risk ratio [RR] 1.00, 95% CI 0.77-1.31, P=0.979). There were no significant differences between BP-SES and DP-EES with respect to cardiac death (RR 1.01, 95% CI 0.62-1.63, P=0.984), target-vessel myocardial infarction (RR 0.91, 95% CI 0.60-1.39, P=0.669), target-lesion revascularization (RR 1.17, 95% CI 0.81-1.71, P=0.403), and definite stent thrombosis (RR 1.38, 95% CI 0.56-3.44, P=0.485). There were 2 cases (0.2%) of definite very late stent thrombosis in the BP-SES arm and 4 cases (0.4%) in the DP-EES arm (P=0.423). In the prespecified subgroup of patients with ST-segment elevation myocardial infarction, BP-SES was associated with a lower risk of target-lesion failure compared with DP-EES (RR 0.48, 95% CI 0.23-0.99, P=0.043, Pinteraction=0.026).
Conclusions: Comparable safety and efficacy profiles of BP-SES and DP-EES were maintained throughout 2 years of follow-up.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT01443104.
Keywords: biodegradable polymer; drug‐eluting stent; everolimus‐eluting stent; percutaneous coronary intervention; sirolimus‐eluting stent.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


References
-
- Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. - PubMed
-
- Piccolo R, Giustino G, Mehran R, Windecker S. Stable coronary artery disease: revascularisation and invasive strategies. Lancet. 2015;386:702–713. - PubMed
-
- Windecker S, Meier B. Late coronary stent thrombosis. Circulation. 2007;116:1952–1965. - PubMed
-
- Stefanini GG, Holmes DR Jr. Drug‐eluting coronary‐artery stents. N Engl J Med. 2013;368:254–265. - PubMed
-
- Piccolo R, Stefanini GG, Franzone A, Spitzer E, Blochlinger S, Heg D, Juni P, Windecker S. Safety and efficacy of resolute zotarolimus‐eluting stents compared with everolimus‐eluting stents: a meta‐analysis. Circ Cardiovasc Interv. 2015;8:e002223. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

