Identification of a Tsal152-75 salivary synthetic peptide to monitor cattle exposure to tsetse flies
- PMID: 26979518
- PMCID: PMC4791801
- DOI: 10.1186/s13071-016-1414-8
Identification of a Tsal152-75 salivary synthetic peptide to monitor cattle exposure to tsetse flies
Abstract
Background: The saliva of tsetse flies contains a cocktail of bioactive molecules inducing specific antibody responses in hosts exposed to bites. We have previously shown that an indirect-ELISA test using whole salivary extracts from Glossina morsitans submorsitans was able to discriminate between (i) cattle from tsetse infested and tsetse free areas and (ii) animals experimentally exposed to low or high numbers of tsetse flies. In the present study, our aim was to identify specific salivary synthetic peptides that could be used to develop simple immunoassays to measure cattle exposure to tsetse flies.
Methods: In a first step, 2D-electrophoresis immunoblotting, using sera from animals exposed to a variety of bloodsucking arthropods, was performed to identify specific salivary proteins recognised in cattle exposed to tsetse bites. Linear epitope prediction software and Blast analysis were then used to design synthetic peptides within the identified salivary proteins. Finally, candidate peptides were tested by indirect-ELISA on serum samples from tsetse infested and tsetse free areas, and from exposure experiments.
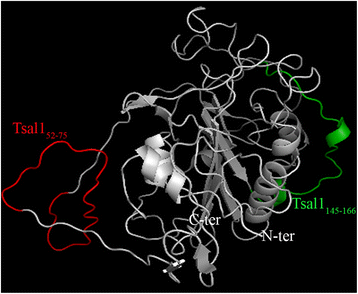
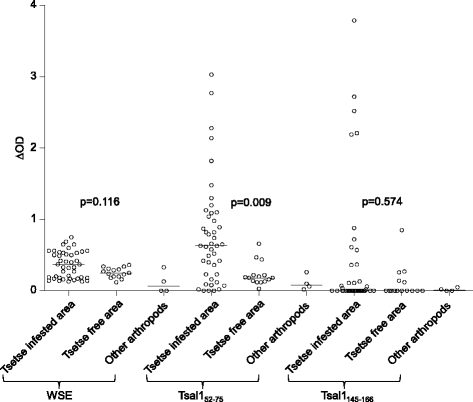
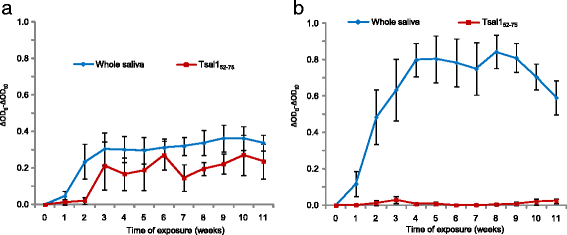
Results: The combined immunoblotting and bioinformatics analyses led to the identification of five peptides carrying putative linear epitopes within two salivary proteins: the tsetse salivary gland protein 1 (Tsal1) and the Salivary Secreted Adenosine (SSA). Of these, two were synthesised and tested further based on the absence of sequence homology with other arthropods or pathogen species. IgG responses to the Tsal152-75 synthetic peptide were shown to be specific of tsetse exposure in both naturally and experimentally exposed hosts. Nevertheless, anti-Tsal152-75 IgG responses were absent in animals exposed to high tsetse biting rates.
Conclusions: These results suggest that Tsal152-75 specific antibodies represent a biomarker of low cattle exposure to tsetse fly. These results are discussed in the light of the other available tsetse saliva based-immunoassays and in the perspective of developing a simple serological tool for tsetse eradication campaigns to assess the tsetse free status or to detect tsetse reemergence in previously cleared areas.
Keywords: African Animal Trypanosomosis; Biomarker of Exposure; Cattle; Synthetic Peptide; Tsetse Flies.
Figures


References
-
- Cattand P. Food and Agriculture Organization, Programme Against African Trypanosomiasis (PAAT). Linking sustainable human and animal African trypanosomiasis control with rural development strategies. 2010. p. 76.
-
- FAO . Impacts of trypanosomosis on African agriculture, by B.M. Swallow. Rome: PAAT Technical and Scientific Series No. 2; 2000.
-
- Feldmann U, Hendrichs J. The concept for integration of the sterile insect technique as a key component of future sub-regional, area-wide tsetse and trypanosomosis management operations. In: Feldmann U, Hendrichs J, editors. Animal Trypanosomosis: Vector and Disease Control Using Nuclear Techniques: Proceeding of the Second FAO/IAEA Seminar for Africa, November 27-December 1, 1995, Zanzibar, United Republic of Tanzania. Vienna: Backhuys Publishers; 1995. pp. 193–214.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

