The green tea polyphenol EGCG alleviates maternal diabetes-induced neural tube defects by inhibiting DNA hypermethylation
- PMID: 26979632
- PMCID: PMC5270539
- DOI: 10.1016/j.ajog.2016.03.009
The green tea polyphenol EGCG alleviates maternal diabetes-induced neural tube defects by inhibiting DNA hypermethylation
Abstract
Background: Maternal diabetes increases the risk of neural tube defects in offspring. Our previous study demonstrated that the green tea polyphenol, Epigallocatechin gallate, inhibits high glucose-induced neural tube defects in cultured embryos. However, the therapeutic effect of Epigallocatechin gallate on maternal diabetes-induced neural tube defects is still unclear.
Objective: We aimed to examine whether Epigallocatechin gallate treatment can reduce maternal diabetes-induced DNA methylation and neural tube defects.
Study design: Nondiabetic and diabetic pregnant mice at embryonic day 5.5 were given drinking water with or without 1 or 10 μM Epigallocatechin gallate. At embryonic day 8.75, embryos were dissected from the visceral yolk sac for the measurement of the levels and activity of DNA methyltransferases, the levels of global DNA methylation, and methylation in the CpG islands of neural tube closure essential gene promoters. embryonic day 10.5 embryos were examined for neural tube defect incidence.
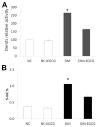
Results: Epigallocatechin gallate treatment did not affect embryonic development because embryos from nondiabetic dams treated with Epigallocatechin gallate did not exhibit any neural tube defects. Treatment with 1 μM Epigallocatechin gallate did not reduce maternal diabetes-induced neural tube defects significantly. Embryos from diabetic dams treated with 10 μM Epigallocatechin gallate had a significantly lower neural tube defect incidence compared with that of embryos without Epigallocatechin gallate treatment. Epigallocatechin gallate reduced neural tube defect rates from 29.5% to 2%, an incidence that is comparable with that of embryos from nondiabetic dams. Ten micromoles of Epigallocatechin gallate treatment blocked maternal diabetes-increased DNA methyltransferases 3a and 3b expression and their activities, leading to the suppression of global DNA hypermethylation. Additionally, 10 μM Epigallocatechin gallate abrogated maternal diabetes-increased DNA methylation in the CpG islands of neural tube closure essential genes, including Grhl3, Pax3, and Tulp3.
Conclusion: Epigallocatechin gallate reduces maternal diabetes-induced neural tube defects formation and blocks the enhanced expression and activity of DNA methyltransferases, leading to the suppression of DNA hypermethylation and the restoration of neural tube closure essential gene expression. These observations suggest that Epigallocatechin gallate supplements could mitigate the teratogenic effects of hyperglycemia on the developing embryo and prevent diabetes-induced neural tube defects.
Keywords: DNA methyltransferases; Epigallocatechin gallate; essential gene; green tea polyphenol; hypermethylation; maternal diabetes; neural tube closure; neural tube defects.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

