Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice
- PMID: 26980205
- PMCID: PMC4872877
- DOI: 10.1093/cvr/cvw054
Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice
Abstract
Aims: Extracellular vesicles (EVs) from mice with monocrotaline (MCT)-induced pulmonary hypertension (PH) induce PH in healthy mice, and the exosomes (EXO) fraction of EVs from mesenchymal stem cells (MSCs) can blunt the development of hypoxic PH. We sought to determine whether the EXO fraction of EVs is responsible for modulating pulmonary vascular responses and whether differences in EXO-miR content explains the differential effects of EXOs from MSCs and mice with MCT-PH.
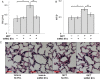
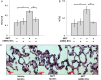
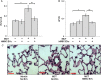
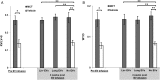
Methods and results: Plasma, lung EVs from MCT-PH, and control mice were divided into EXO (exosome), microvesicle (MV) fractions and injected into healthy mice. EVs from MSCs were divided into EXO, MV fractions and injected into MCT-treated mice. PH was assessed by right ventricle-to-left ventricle + septum (RV/LV + S) ratio and pulmonary arterial wall thickness-to-diameter (WT/D) ratio. miR microarray analyses were also performed on all EXO populations. EXOs but not MVs from MCT-injured mice increased RV/LV + S, WT/D ratios in healthy mice. MSC-EXOs prevented any increase in RV/LV + S, WT/D ratios when given at the time of MCT injection and reversed the increase in these ratios when given after MCT administration. EXOs from MCT-injured mice and patients with idiopathic pulmonary arterial hypertension (IPAH) contained increased levels of miRs-19b,-20a,-20b, and -145, whereas miRs isolated from MSC-EXOs had increased levels of anti-inflammatory, anti-proliferative miRs including miRs-34a,-122,-124, and -127.
Conclusion: These findings suggest that circulating or MSC-EXOs may modulate pulmonary hypertensive effects based on their miR cargo. The ability of MSC-EXOs to reverse MCT-PH offers a promising potential target for new PAH therapies.
Keywords: Exosomes; Extracellular vesicles; Mesenchymal stem cells; MicroRNA; Pulmonary hypertension.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2016. For permissions please email: journals.permissions@oup.com.
Figures


Comment in
-
Beware of the content!-exosomes as benefactors and agitators.Cardiovasc Res. 2016 Jun 1;110(3):293-4. doi: 10.1093/cvr/cvw088. Epub 2016 Apr 21. Cardiovasc Res. 2016. PMID: 27102217 No abstract available.
References
-
- Mathew R. Pathogenesis of pulmonary hypertension: a case for caveolin-1 and cell membrane integrity. Am J Physiol Heart Circ Physiol 2014;306:H15–H25. - PubMed
-
- Bienertova-Vasku J, Novak J, Vasku A. MicroRNAs in pulmonary arterial hypertension: pathogenesis, diagnosis and treatment. J Am Soc Hypertens 2015;9:221–234. - PubMed
-
- Guignabert C, Tu L, Girerd B, Ricard N, Huertas A, Montani D, Humbert M. New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication. Chest 2015;147:529–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

