Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects
- PMID: 26980293
- PMCID: PMC4792107
- DOI: 10.1186/s12967-016-0825-9
Advantages of pure platelet-rich plasma compared with leukocyte- and platelet-rich plasma in promoting repair of bone defects
Abstract
Background: High levels of pro-inflammatory cytokines in leukocyte- and platelet-rich plasma (L-PRP) may activate the nuclear factor κB (NF-κB) pathway to counter the beneficial effect of the growth factors on bone regeneration. However, to date, no relevant studies have substantiated this.
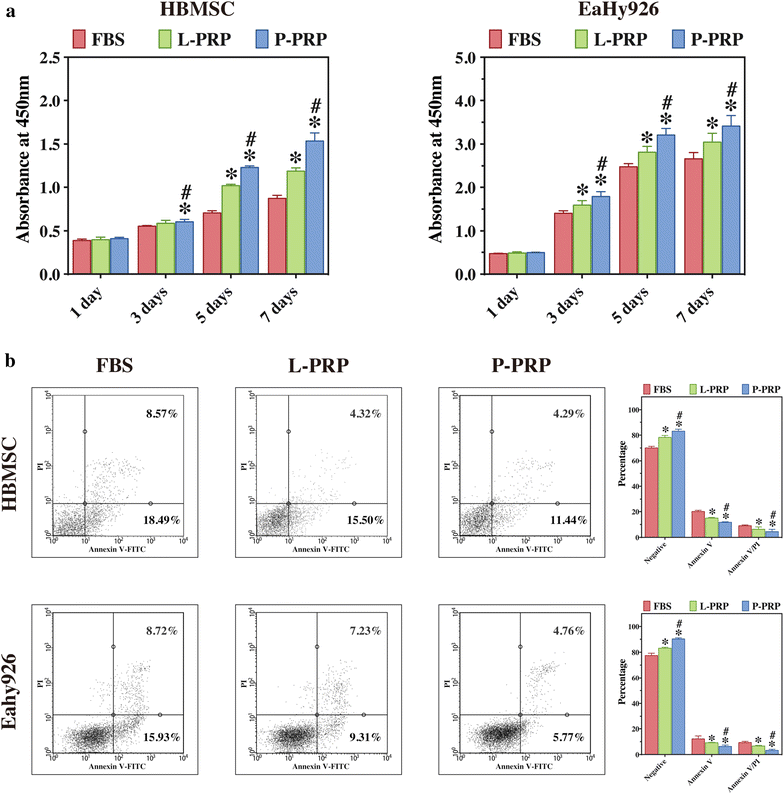
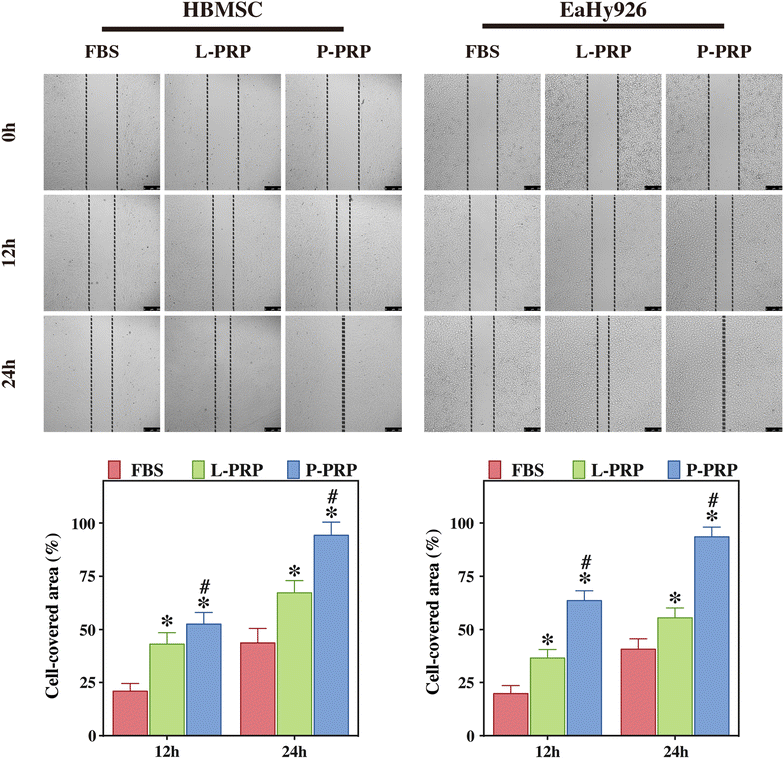
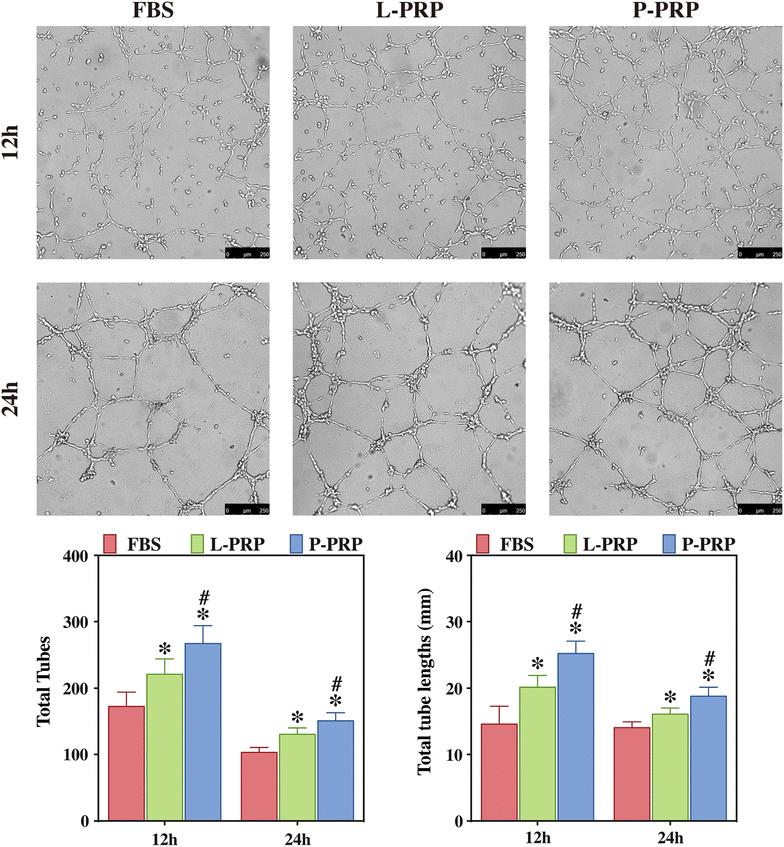
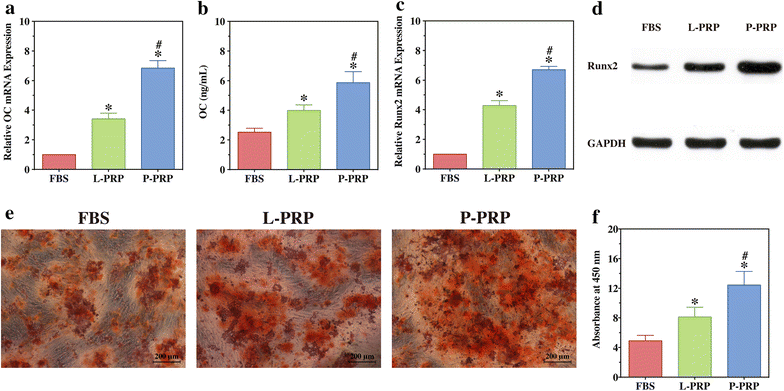
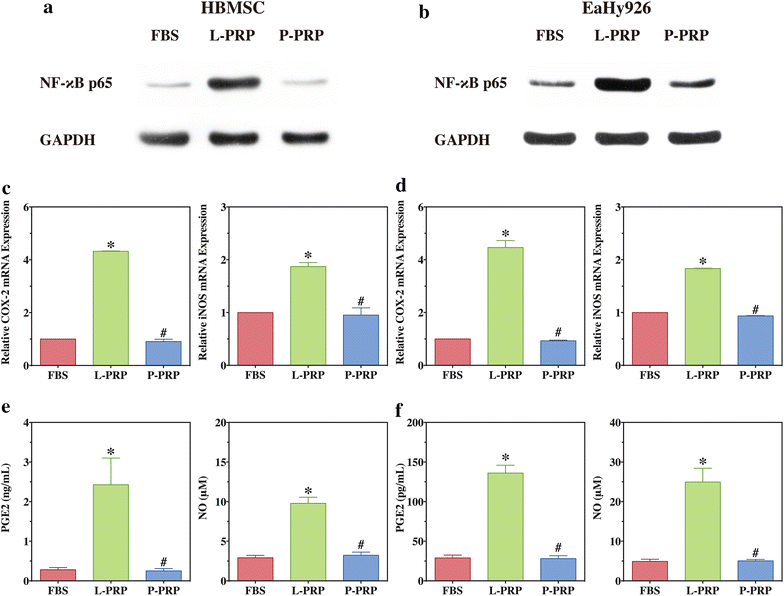
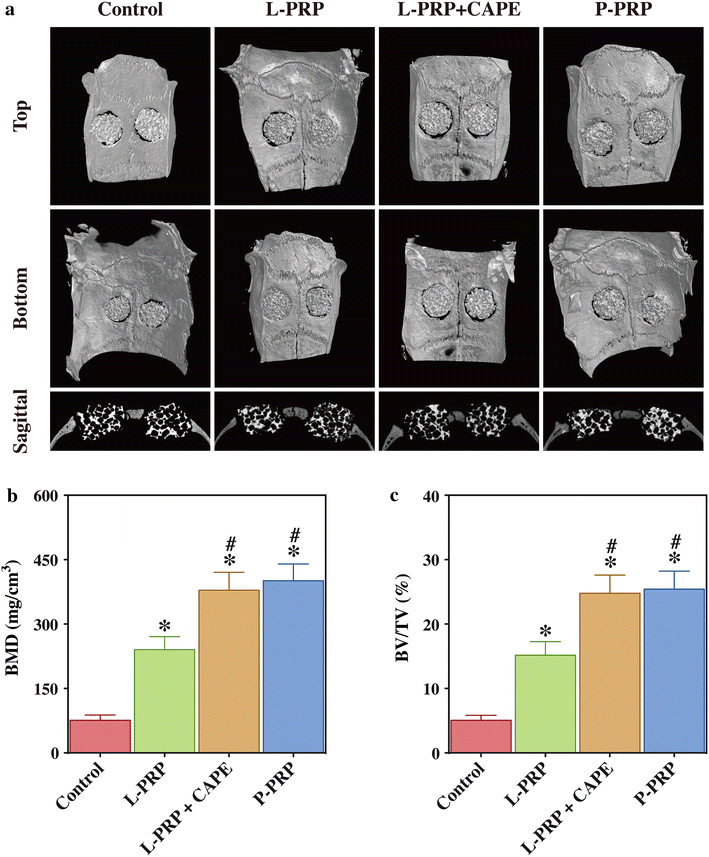
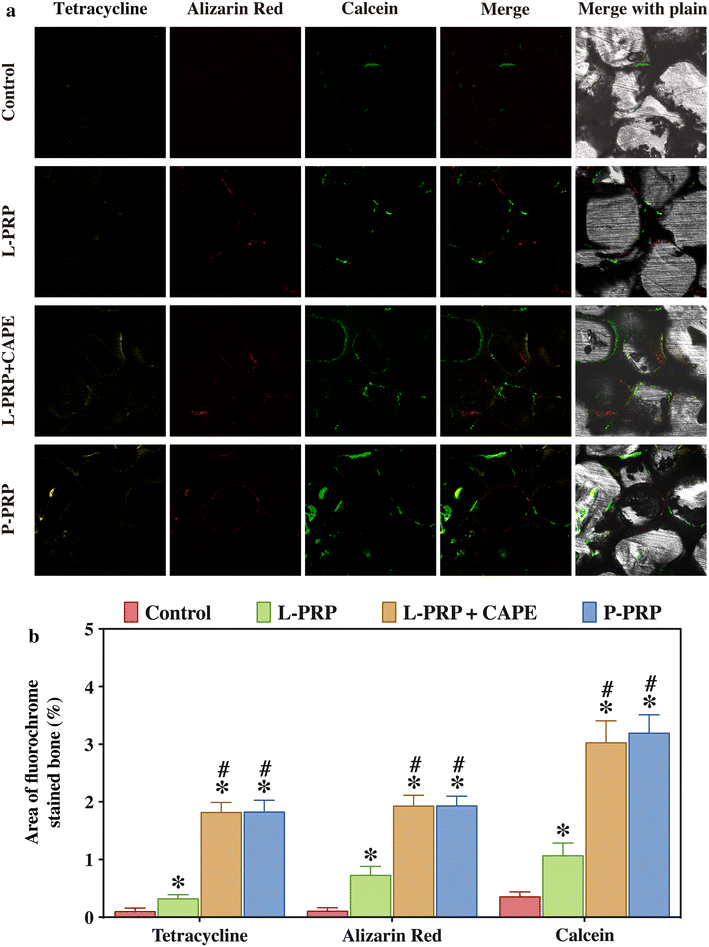
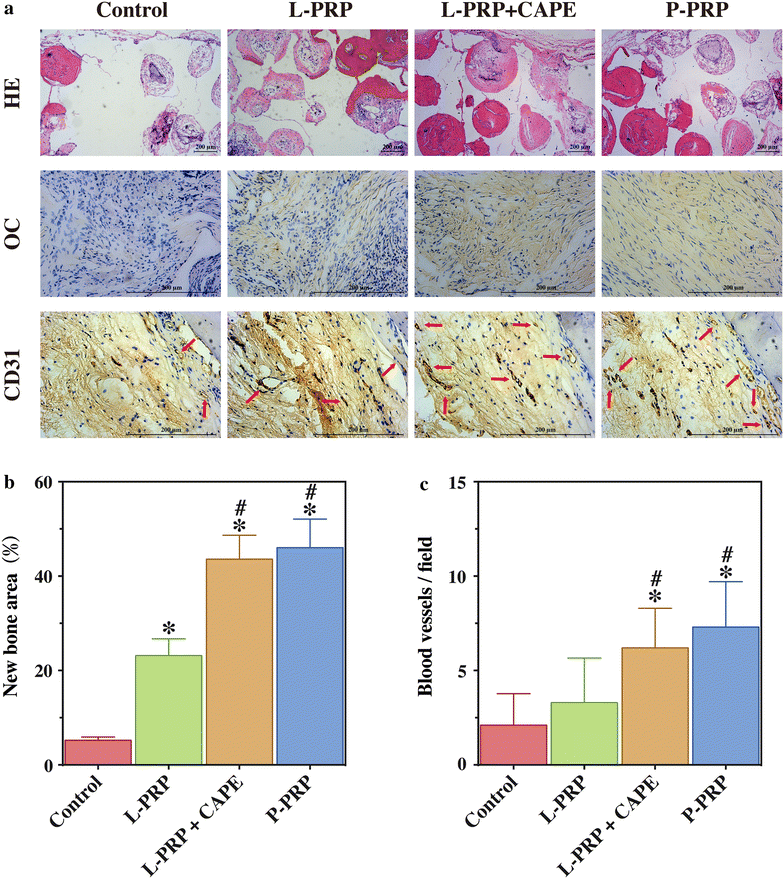
Methods: L-PRP and pure platelet-rich plasma (P-PRP) were isolated. The in vitro effects of L-PRP and P-PRP on the proliferation, viability and migration of human bone marrow-derived mesenchymal stem cells (HBMSCs) and EaHy926, tube formation of EaHy926, and osteogenic differentiation of HBMSCs were assessed by cell counting, flow cytometry, scratch assay, tube formation assay, and real-time quantitative polymerase chain reaction (RT-PCR), western blotting and Alizarin red staining, respectively. The in vitro effects of L-PRP and P-PRP on the nuclear translocation of NF-κB p65, mRNA expression of inducible nitric oxide synthase and cyclooxygenase-2, and production of prostaglandin E2 and nitric oxid were assessed by western blotting, RT-PCR, enzyme-linked immunosorbent assay and Griess reaction, respectively. The in vivo effects of L-PRP or P-PRP preprocessed β-tricalcium phosphate (β-TCP) on the calvarial defects in rats were assessed by histological and immunofluorescence examinations.
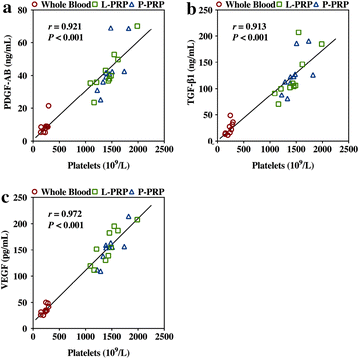
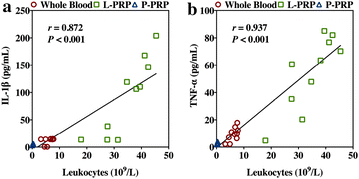
Results: P-PRP, which had similar platelet and growth factors concentrations but significantly lower concentrations of leukocytes and pro-inflammatory cytokines compared with L-PRP, promoted the proliferation, viability and migration of HBMSCs and EaHy926, tube formation of EaHy926 and osteogenic differentiation of HBMSCs in vitro, compared with L-PRP. The implantation of P-PRP preprocessed β-TCP also yielded better histological results than the implantation of L-PRP preprocessed β-TCP in vivo. Moreover, L-PRP treatment resulted in the activation of the NF-κB pathway in HBMSCs and EaHy926 in vitro while the postoperative delivery of caffeic acid phenethyl ester, an inhibitor of NF-κB activation, enhanced the histological results of the implantation of L-PRP preprocessed β-TCP in vivo.
Conclusions: Leukocytes in L-PRP may activate the NF-κB pathway via the increased pro-inflammatory cytokines to induce the inferior effects on bone regeneration of L-PRP compared with P-PRP. Hence, P-PRP may be more suitable for bone regeneration compared with L-PRP, and the combined use of P-PRP and β-TCP represents a safe, simple, and effective alternative option for autogenous bone graft in the treatment of bone defects.
Keywords: Animal model; Bone regeneration; Leukocyte- and platelet-rich plasma; Nuclear factor κB; Platelet-rich plasma; Pure platelet-rich plasma.
Figures
References
-
- Hirota M, Matsui Y, Mizuki N, Kishi T, Watanuki K, Ozawa T, et al. Combination with allogenic bone reduces early absorption of beta-tricalcium phosphate (beta-TCP) and enhances the role as a bone regeneration scaffold. Experimental animal study in rat mandibular bone defects. Dent Mater J. 2009;28:153–161. doi: 10.4012/dmj.28.153. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

