Basal levels of AMPA receptor GluA1 subunit phosphorylation at threonine 840 and serine 845 in hippocampal neurons
- PMID: 26980779
- PMCID: PMC4793196
- DOI: 10.1101/lm.040675.115
Basal levels of AMPA receptor GluA1 subunit phosphorylation at threonine 840 and serine 845 in hippocampal neurons
Abstract
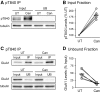
Dephosphorylation of AMPA receptor (AMPAR) GluA1 subunits at two sites, serine 845 (S845) and threonine 840 (T840), is thought to be involved in NMDA receptor-dependent forms of long-term depression (LTD). Importantly, the notion that dephosphorylation of these sites contributes to LTD assumes that a significant fraction of GluA1 subunits are basally phosphorylated at these sites. To examine this question, we used immunoprecipitation/depletion assays to estimate the proportion of GluA1 subunits basally phosphorylated at S845 and T840. Although dephosphorylation of S845 is thought to have a key role in LTD, our results indicate that few GluA1 subunits in hippocampal neurons are phosphorylated at this site. In contrast, ∼50% of GluA1 subunits are basally phosphorylated at T840, suggesting that dephosphorylation of this site can contribute to the down-regulation of AMPAR-mediated synaptic transmission in LTD.
© 2016 Babiec et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
-
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, et al. 2006. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics 5: 1158–1170. - PubMed
-
- Derkach VA, Oh MC, Guire ES, Soderling TR. 2007. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8: 101–113. - PubMed
